Pathogenic bacteria
| Pathogenic bacteria | |
|---|---|
 _and_YLLs_(B)%252C_by_pathogen_and_GBD_super-region%252C_2019.jpg.webp) | |
|
| |
| Specialty | Infectious disease |
Pathogenic bacteria are bacteria that can cause disease.[2] This article focuses on the bacteria that are pathogenic to humans. Most species of bacteria are harmless and are often beneficial but others can cause infectious diseases. The number of these pathogenic species in humans is estimated to be fewer than a hundred.[3] By contrast, several thousand species are part of the gut flora present in the digestive tract.
The body is continually exposed to many species of bacteria, including beneficial commensals, which grow on the skin and mucous membranes, and saprophytes, which grow mainly in the soil and in decaying matter. The blood and tissue fluids contain nutrients sufficient to sustain the growth of many bacteria. The body has defence mechanisms that enable it to resist microbial invasion of its tissues and give it a natural immunity or innate resistance against many microorganisms.
Pathogenic bacteria are specially adapted and endowed with mechanisms for overcoming the normal body defences, and can invade parts of the body, such as the blood, where bacteria are not normally found. Some pathogens invade only the surface epithelium, skin or mucous membrane, but many travel more deeply, spreading through the tissues and disseminating by the lymphatic and blood streams. In some rare cases a pathogenic microbe can infect an entirely healthy person, but infection usually occurs only if the body's defence mechanisms are damaged by some local trauma or an underlying debilitating disease, such as wounding, intoxication, chilling, fatigue, and malnutrition. In many cases, it is important to differentiate infection and colonization, which is when the bacteria are causing little or no harm.
Caused by Mycobacterium tuberculosis bacteria, one of the diseases with the highest disease burden is tuberculosis, which killed 1.4 million people in 2019, mostly in sub-Saharan Africa.[4] Pathogenic bacteria contribute to other globally important diseases, such as pneumonia, which can be caused by bacteria such as Staphylococcus, Streptococcus and Pseudomonas, and foodborne illnesses, which can be caused by bacteria such as Shigella, Campylobacter, and Salmonella. Pathogenic bacteria also cause infections such as tetanus, typhoid fever, diphtheria, syphilis, and leprosy.
Pathogenic bacteria are also the cause of high infant mortality rates in developing countries.[5] A GBD study estimated the global death rates from (33) bacterial pathogens, finding such infections contributed to one in 8 deaths (or ~7.7 million deaths), which could make it the second largest cause of death globally in 2019.[6][1]
Most pathogenic bacteria can be grown in cultures and identified by Gram stain and other methods. Bacteria grown in this way are often tested to find which antibiotics will be an effective treatment for the infection. For hitherto unknown pathogens, Koch's postulates are the standard to establish a causative relationship between a microbe and a disease.
Diseases
Each species has specific effect and causes symptoms in people who are infected. Some people who are infected with a pathogenic bacteria do not have symptoms. Immunocompromised individuals are more susceptible to pathogenic bacteria.[7]
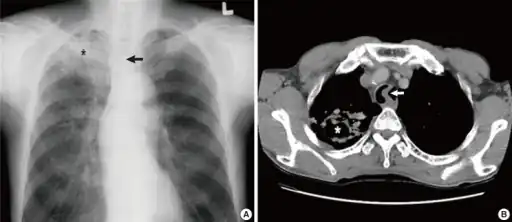 a)Image reveals tuberculosis asterisk b) CT scan of the chest shows tuberculosis with cavitation asterisk
a)Image reveals tuberculosis asterisk b) CT scan of the chest shows tuberculosis with cavitation asterisk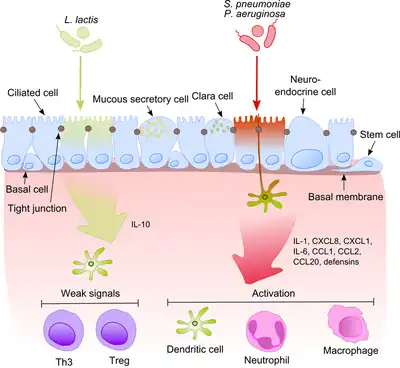 Commensals vs pathogenic bacteria in COPD
Commensals vs pathogenic bacteria in COPD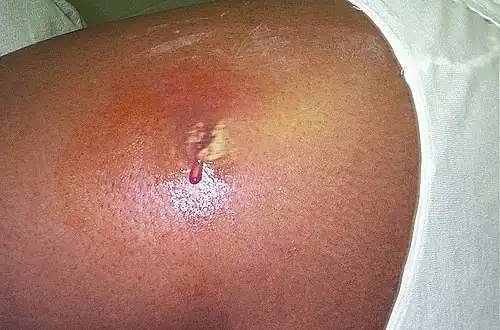 An abscess caused by opportunistic S. aureus bacteria.
An abscess caused by opportunistic S. aureus bacteria.
Pathogenic susceptibility
Some pathogenic bacteria cause disease under certain conditions, such as entry through the skin via a cut, through sexual activity or through a compromised immune function.
Some species of Streptococcus and Staphylococcus are part of the normal skin microbiota and typically reside on healthy skin or in the nasopharangeal region. Yet these species can potentially initiate skin infections. Streptoccal infections include sepsis, pneumonia, and meningitis.[8] These infections can become serious creating a systemic inflammatory response resulting in massive vasodilation, shock, and death.[9]
Other bacteria are opportunistic pathogens and cause disease mainly in people with immunosuppression or cystic fibrosis. Examples of these opportunistic pathogens include Pseudomonas aeruginosa, Burkholderia cenocepacia, and Mycobacterium avium.[10][11]
Intracellular
Obligate intracellular parasites (e.g. Chlamydophila, Ehrlichia, Rickettsia) have the ability to only grow and replicate inside other cells. Even these intracellular infections may be asymptomatic, requiring an incubation period. An example of this is Rickettsia which causes typhus. Another causes Rocky Mountain spotted fever.
Chlamydia are intracellular parasites. These pathogens can cause pneumonia or urinary tract infection and may be involved in coronary heart disease.[12]
Other groups of intracellular bacterial pathogens include Salmonella, Neisseria, Brucella, Mycobacterium, Nocardia, Listeria, Francisella, Legionella, and Yersinia pestis. These can exist intracellularly, but can exist outside of host cells.
Infections in specific tissue
Bacterial pathogens often cause infection in specific areas of the body. Others are generalists.
- Bacterial vaginosis is a condition of the vaginal microbiota in which an excessive growth of Gardnerella vaginalis and other mostly anaerobic bacteria displace the beneficial Lactobacilli species that maintain healthy vaginal microbial populations.[13]
- Bacterial meningitis is a bacterial inflammation of the meninges, which are the protective membranes covering the brain and spinal cord.
- Bacterial pneumonia is a bacterial infection of the lungs.
- Urinary tract infection is predominantly caused by bacteria. Symptoms include the strong and frequent sensation or urge to urinate, pain during urination, and urine that is cloudy.[14] The most frequent cause is Escherichia coli. Urine is typically sterile but contains a variety of salts, and waste products.[15] Bacteria can ascend into the bladder or kidney and causing cystitis and nephritis.
- Bacterial gastroenteritis is caused by enteric, pathogenic bacteria. These pathogenic species are usually distinct from the usually harmless bacteria of the normal gut flora. But a different strain of the same species may be pathogenic. The distinction is sometimes difficult as in the case of Escherichia.
- Bacterial skin infections include:
- Impetigo is a highly contagious bacterial skin infection commonly seen in children.[16] It is caused by Staphylococcus aureus, and Streptococcus pyogenes.[17]
- Erysipelas is an acute streptococcus bacterial infection[18] of the deeper skin layers that spreads via with lymphatic system.
- Cellulitis is a diffuse inflammation[19] of connective tissue with severe inflammation of dermal and subcutaneous layers of the skin. Cellulitis can be caused by normal skin flora or by contagious contact, and usually occurs through open skin, cuts, blisters, cracks in the skin, insect bites, animal bites, burns, surgical wounds, intravenous drug injection, or sites of intravenous catheter insertion. In most cases it is the skin on the face or lower legs that is affected, though cellulitis can occur in other tissues.
Mechanisms of damage
The symptoms of disease appear as pathogenic bacteria damage host tissues or interfere with their function. The bacteria can damage host cells directly or indirectly by provoking an immune response that inadvertently damages host cells,[20] or by releasing toxins.[21]
Direct
Once pathogens attach to host cells, they can cause direct damage as the pathogens use the host cell for nutrients and produce waste products.[22] For example, Streptococcus mutans, a component of dental plaque, metabolizes dietary sugar and produces acid as a waste product. The acid decalcifies the tooth surface to cause dental caries.[23]
Toxin production

Endotoxins are the lipid portions of lipopolysaccharides that are part of the outer membrane of the cell wall of gram-negative bacteria. Endotoxins are released when the bacteria lyses, which is why after antibiotic treatment, symptoms can worsen at first as the bacteria are killed and they release their endotoxins. Exotoxins are secreted into the surrounding medium or released when the bacteria die and the cell wall breaks apart.[24]
Indirect
An excessive or inappropriate immune response triggered by an infection may damage host cells.[2]
Survival in host
Nutrients
Iron is required for humans, as well as the growth of most bacteria. To obtain free iron, some pathogens secrete proteins called siderophores, which take the iron away from iron-transport proteins by binding to the iron even more tightly. Once the iron-siderophore complex is formed, it is taken up by siderophore receptors on the bacterial surface and then that iron is brought into the bacterium.[24]
Bacterial pathogens also require access to carbon and energy sources for growth. To avoid competition with host cells for glucose which is the main energy source used by human cells, many pathogens including the respiratory pathogen Haemophilus influenzae specialise in using other carbon sources such as lactate that are abundant in the human body [25]
Identification
Typically identification is done by growing the organism in a wide range of cultures which can take up to 48 hours. The growth is then visually or genomically identified. The cultured organism is then subjected to various assays to observe reactions to help further identify species and strain.[26]
Treatment
Bacterial infections may be treated with antibiotics, which are classified as bacteriocidal if they kill bacteria or bacteriostatic if they just prevent bacterial growth. There are many types of antibiotics and each class inhibits a process that is different in the pathogen from that found in the host. For example, the antibiotics chloramphenicol and tetracyclin inhibit the bacterial ribosome but not the structurally different eukaryotic ribosome, so they exhibit selective toxicity.[27] Antibiotics are used both in treating human disease and in intensive farming to promote animal growth. Both uses may be contributing to the rapid development of antibiotic resistance in bacterial populations.[28] Phage therapy, using bacteriophages can also be used to treat certain bacterial infections.[29]
Prevention
Infections can be prevented by antiseptic measures such as sterilizing the skin prior to piercing it with the needle of a syringe and by proper care of indwelling catheters. Surgical and dental instruments are also sterilized to prevent infection by bacteria. Disinfectants such as bleach are used to kill bacteria or other pathogens on surfaces to prevent contamination and further reduce the risk of infection. Bacteria in food are killed by cooking to temperatures above 73 °C (163 °F).
List of genera and microscopy features
Many genera contain pathogenic bacterial species. They often possess characteristics that help to classify and organize them into groups. The following is a partial listing.
| Genus | Species | Gram staining | Shape | Oxygen requirement | Intra/Extracellular |
|---|---|---|---|---|---|
| Bacillus[30] | Positive | Rods | Facultative anaerobic | Extracellular | |
| Bartonella[30] | Negative | Rods | Aerobic | Facultative intracellular | |
| Bordetella[30] | Negative | Small coccobacilli | Aerobic | Extracellular | |
| Borrelia[30] | Negative, stains poorly | Spirochete | Anaerobic | Extracellular | |
| Brucella[30] | Negative | Coccobacilli | Aerobic | Intracellular | |
| Campylobacter[30] | Negative | Spiral rods[33] coccoid in older cultures[33] |
Microaerophilic[33] | Extracellular | |
| Chlamydia and Chlamydophila[30] | (not Gram-stained) | Small, round, ovoid | Facultative or strictly aerobic | Obligate intracellular | |
| Clostridium[30] | Positive | Large, blunt-ended rods | Obligate anaerobic | Extracellular | |
| Corynebacterium[30] | Positive (unevenly) | Rods | Mostly facultative anaerobic | Extracellular | |
| Enterococcus[32][36] | Positive | Cocci | Facultative Anaerobic | Extracellular | |
| Escherichia[5][32][37] | Negative | Rods | Facultative anaerobic | Extracellular or Intracellular | |
| Francisella[30] | Negative | Coccobacillus | Strictly aerobic | Facultative intracellular | |
| Haemophilus | Negative | Coccobacilli to long and slender filaments | Facultative anaerobic 5 - 10% CO2 | Extracellular | |
| Helicobacter | Negative | Spiral rod | Microaerophile | Extracellular | |
| Legionella[30] | Negative, stains poorly | Cocobacilli | Aerobic | Facultative intracellular | |
| Leptospira[32][40] | Negative, stains poorly | Spirochete | Strictly aerobic | Extracellular | |
| Listeria[30] | Positive, darkly | Slender, short rods | Facultative Anaerobic | Facultative intracellular | |
| Mycobacterium[30] | (none) | Long, slender rods | Aerobic | Intracellular | |
| Mycoplasma[30] | (none) | Indistinct 'fried egg' appearance, no cell wall | Mostly facultative anaerobic; M. pneumoniae strictly aerobic | Extracellular | |
| Neisseria[32][41] | Negative | Kidney bean-shaped | Aerobic | Gonococcus: facultative intracellular N. meningitidis: extracellular | |
| Pseudomonas[32][42] | Negative | Rods | Obligate aerobic | Extracellular | |
| Rickettsia[30] | Negative, stains poorly | Small, rod-like coccobacillary | Aerobic | Obligate intracellular | |
| Salmonella[30] | Negative | Rods | Facultative anaerobica | Facultative intracellular | |
| Shigella[32][43] | Negative | Rods | Facultative anaerobic | Extracellular | |
| Staphylococcus[5] | Positive, darkly | Round cocci | Facultative anaerobic | Extracellular, facultative intracellular | |
| Streptococcus[30] | Positive | Ovoid to spherical | Facultative anaerobic | Extracellular | |
| Treponema[30] | Negative, stains poorly | Spirochete | Aerobic | Extracellular | |
| Ureaplasma[5] | Stains poorly[44] | Indistinct, 'fried egg' appearance, no cell wall | Anaerobic | Extracellular | |
| Vibrio[32][45] | Negative | Spiral with single polar flagellum | Facultative anaerobic | Extracellular | |
| Yersinia[32][46] | Negative, bipolarly | Small rods | Facultative anaerobe | Intracellular | |
List of species and clinical characteristics
 Overall age-standardised mortality rate per 100 000 population for 33 pathogens investigated, 2019[1]
Overall age-standardised mortality rate per 100 000 population for 33 pathogens investigated, 2019[1]_and_YLLs_(B)%252C_by_pathogen_and_infectious_syndrome%252C_2019.jpg.webp) Global number of deaths (A) and YLLs (B), by pathogen and infectious syndrome, 2019[1]
Global number of deaths (A) and YLLs (B), by pathogen and infectious syndrome, 2019[1]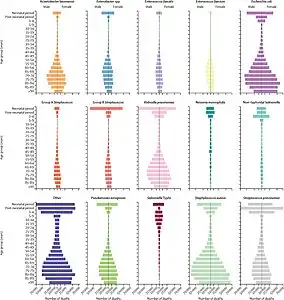 Global number of deaths, by pathogen, age, and sex groups, 2019[1]
Global number of deaths, by pathogen, age, and sex groups, 2019[1]
This is description of the more common genera and species presented with their clinical characteristics and treatments.
| Species | Transmission | Diseases | Treatment | Prevention | ||
|---|---|---|---|---|---|---|
| Actinomyces israelii | Oral flora[47] | Actinomycosis:[47] painful abscesses and cysts MRSA in the mouth, lungs,[48][49] or gastrointestinal tract.[34] | Prolonged penicillin G and drainage[47] | |||
| Bacillus anthracis |
Contact with cattle, sheep, goats and horses[50] |
Anthrax: pulmonary, gastrointestinal and/or cutaneous symptoms.[47] |
In early infection:[51] |
Anthrax vaccine[32] | ||
| Bacteroides fragilis | Gut flora[47] | Abscesses in gastrointestinal tract, pelvic cavity and lungs[47] | metronidazole[47] | Wound care[53] | ||
| Bordetella pertussis |
Contact with respiratory droplets expelled by infected human hosts.[32] |
Macrolides[32] such as erythromycin,[32][47] before paroxysmal stage[47] |
Pertussis vaccine,[32][47] such as in DPT vaccine[32][47] | |||
| Borrelia | B. burgdorferi[32][47] |
Ixodes hard ticks |
|
Doxycycline for adults, amoxicillin for children, ceftriaxone for neurological involvement[55] |
Wearing clothing that limits skin exposure to ticks.[32] | |
| B. recurrentis[57] and others[note 1] |
Pediculus humanus corporis body louse (B. recurrentis only) and Ornithodoros soft ticks[57] | Relapsing fever | Penicillin, tetracycline, doxycycline[58] | Avoid areas where ticks are found[57] Better access to washing facilities[57] | ||
| Brucella | B. abortus |
Direct contact with infected animal[32] |
Brucellosis: mainly fever, muscular pain and night sweats |
|||
| Campylobacter jejuni |
Fecal-oral from animals (mammals and fowl)[32][47] |
|
Treat symptoms[32] |
Good hygiene[32] | ||
| Chlamydia | C. pneumoniae | Atypical pneumonia[47] | None[32] | |||
| C. trachomatis |
vaginal sex[32] |
Trachoma[32][47] |
Erythromycin[32][47] |
Erythromycin or silver nitrate in newborn's eyes[32] | ||
| Chlamydophila psittaci | Inhalation of dust with secretions or feces from birds (e.g. parrots) | Psittacosis, mainly atypical pneumonia | - | |||
| Clostridium | C. botulinum | Spores from soil,[32][47] persevere in canned food, smoked fish and honey[47] |
Botulism: Mainly muscle weakness and paralysis[47] |
Antitoxin[32][47] |
Proper food preservation techniques | |
| C. difficile |
Gut flora,[32][47] overgrowing when other flora is depleted[32] |
Discontinuing responsible antibiotic[32][47] |
Fecal bacteriotherapy | |||
| C. perfringens |
Spores in soil[32][47] |
Anaerobic cellulitis[32][47] |
Gas gangrene:
Debridement or amputation[32][47] |
Appropriate food handling[32] | ||
| C. tetani |
Tetanus immune globulin[32][47]
Sedatives[32] |
Tetanus vaccine (such as in the DPT vaccine)[32] | ||||
| Corynebacterium diphtheriae |
respiratory droplets |
Diphtheria: Fever, sore throat and neck swelling, potentially narrowing airways.[60] |
Horse serum antitoxin |
|||
| Ehrlichia | E. canis[47] |
Dog tick[47] | Ehrlichiosis:[47] headache, muscle aches, and fatigue | |||
| Enterococcus | E. faecalis |
Part of gut flora,[47] opportunistic or entering through GI tract or urinary system wounds[32] |
Bacterial endocarditis,[47] biliary tract infections,[47] urinary tract infections[47] |
Ampicillin (combined with aminoglycoside in endocarditis)[47] Vancomycin[32] |
No vaccine Hand washing and other nosocomial prevention | |
| Escherichia | E. coli (generally) |
|
UTI:[32]
(resistance-tests are required first) Meningitis:[32]
Diarrhea:[32]
|
(no vaccine or preventive drug)[32] | ||
| Enterotoxigenic E. coli (ETEC) |
|
|||||
| Enteropathogenic E. coli |
| |||||
| Enteroinvasive E.coli (EIEC) |
| |||||
| Enterohemorrhagic (EHEC), including E. coli O157:H7 |
|
|||||
| Francisella tularensis | Tularemia: Fever, ulceration at entry site and/or lymphadenopathy.[62] Can cause severe pneumonia.[62] | |||||
| Haemophilus influenzae | Meningitis:[32]
(resistance-tests are required first)
|
| ||||
| Helicobacter pylori |
|
|
(No vaccine or preventive drug)[32] | |||
| Klebsiella pneumoniae |
|
|
||||
| Legionella pneumophila | (no vaccine or preventive drug)[32]
Heating water[32] | |||||
| Leptospira species |
|
|
|
Vaccine not widely used[32]
Prevention of exposure[32]
| ||
| Listeria monocytogenes | (no vaccine)[32]
| |||||
| Mycobacterium | M. leprae |
|
|
Tuberculoid form:
Lepromatous form: |
| |
| M. tuberculosis |
|
|
(difficult, see Tuberculosis treatment for more details)[32] Standard "short" course:[32]
|
|||
| Mycoplasma pneumoniae |
|
|||||
| Neisseria | N. gonorrhoeae |
|
|
Uncomplicated gonorrhea:[32]
Ophthalmia neonatorum: |
(No vaccine)[32]
| |
| N. meningitidis |
|
|||||
| Pseudomonas aeruginosa | Opportunistic;[47] Infects damaged tissues or people with immunodeficiency.[32] | Pseudomonas infection:[32]
|
(no vaccine)[32]
| |||
| Nocardia asteroides | In soil[47] | Nocardiosis:[47] Pneumonia, endocarditis, keratitis, neurological or lymphocutaneous infection | TMP/SMX[47] | |||
| Rickettsia rickettsii | (no preventive drug or approved vaccine)[32]
| |||||
| Salmonella | S typhi |
|
|
|||
| Other Salmonella species
|
|
(No vaccine or preventive drug)[32] | ||||
| Shigella | S. sonnei[32] |
|
|
|||
| Staphylococcus | aureus | Coagulase-positive staphylococcal infections: |
|
(no vaccine or preventive drug)
| ||
| epidermidis | Human flora in skin,[32][47] anterior nares[32] and mucous membranes[47] |
|
None[32] | |||
| saprophyticus | Part of normal vaginal flora[32] | None[32] | ||||
| Streptococcus | agalactiae | Human flora in vagina,[32][47] urethral mucous membranes,[32] rectum[32]
|
|
|
None[32] | |
| pneumoniae |
|
|
||||
| pyogenes |
|
No vaccine[32]
| ||||
| viridans | Oral flora,[47] penetration through abrasions |
|
Penicillin G[47] | |||
| Treponema pallidum subspecies pallidum |
|
|||||
| Vibrio cholerae |
|
|
| |||
| Yersinia pestis | Plague: |
|
|
|||
Genetic transformation
Of the 59 species listed in the table with their clinical characteristics, 11 species (or 19%) are known to be capable of natural genetic transformation.[80] Natural transformation is a bacterial adaptation for transferring DNA from one cell to another. This process includes the uptake of exogenous DNA from a donor cell by a recipient cell and its incorporation into the recipient cell's genome by recombination. Transformation appears to be an adaptation for repairing damage in the recipient cell's DNA. Among pathogenic bacteria, transformation capability likely serves as an adaptation that facilitates survival and infectivity.[80] The pathogenic bacteria able to carry out natural genetic transformation (of those listed in the table) are Campylobacter jejuni, Enterococcus faecalis, Haemophilus influenzae, Helicobacter pylori, Klebsiella pneumoniae, Legionella pneumophila, Neisseria gonorrhoeae, Neisseria meningitidis, Staphylococcus aureus, Streptococcus pneumoniae and Vibrio cholerae.
See also
Notes
- ↑ Relapsing fever can also be caused by the following Borrelia species: B. crocidurae, B. duttonii, B. hermsii, B. hispanica, B. miyamotoi, B. persica, B. turicatae and B. venezuelensis.
- Barbour, Alan G. (2017). "Relapsing Fever". In Kasper, Dennis L.; Fauci, Anthony S. (eds.). Harrison's Infectious Diseases (3rd ed.). New York: McGraw Hill Education. pp. 678–687. ISBN 978-1-259-83597-1.
References
- 1 2 3 4 5 Ikuta, Kevin S.; Swetschinski, Lucien R.; Aguilar, Gisela Robles; Sharara, Fablina; Mestrovic, Tomislav; Gray, Authia P.; Weaver, Nicole Davis; Wool, Eve E.; et al. (21 November 2022). "Global mortality associated with 33 bacterial pathogens in 2019: a systematic analysis for the Global Burden of Disease Study 2019". The Lancet. 400 (10369): 2221–2248. doi:10.1016/S0140-6736(22)02185-7. ISSN 0140-6736. PMC 9763654. PMID 36423648.
{{cite journal}}: Check|pmc=value (help) - 1 2 Ryan, Kenneth J.; Ray, C. George; Ahmad, Nafees; Drew, W. Lawrence; Lagunoff, Michael; Pottinger, Paul; Reller, L. Barth; Sterling, Charles R. (2014). "Pathogenesis of Bacterial Infections". Sherris Medical Microbiology (6th ed.). New York: McGraw Hill Education. pp. 391–406. ISBN 978-0-07-181826-1.
- ↑ McFall-Ngai, Margaret (2007-01-11). "Adaptive Immunity: Care for the community". Nature. 445 (7124): 153. Bibcode:2007Natur.445..153M. doi:10.1038/445153a. ISSN 0028-0836. PMID 17215830. S2CID 9273396.
- ↑ "Tuberculosis (TB)". www.who.int. Archived from the original on 2013-12-30. Retrieved 2023-02-17.
- 1 2 3 4 Santosham, Mathuram; Chan, Grace J.; Lee, Anne CC; Baqui, Abdullah H.; Tan, Jingwen; Black, Robert E. (2013). "Risk of Early-Onset Neonatal Infection with Maternal Infection or Colonization: A Global Systematic Review and Meta-Analysis". PLOS Medicine. 10 (8): e1001502. doi:10.1371/journal.pmed.1001502. ISSN 1549-1676. PMC 3747995. PMID 23976885.
- ↑ Hou, Chia-Yi (23 November 2022). "Bacterial infections linked to 1 in 8 deaths in 2019". The Hill. Archived from the original on 12 December 2022. Retrieved 12 December 2022.
- ↑ Azoulay E, Russell L, Van de Louw A, Metaxa V, Bauer P, Povoa P, Montero JG, Loeches IM, Mehta S, Puxty K, Schellongowski P, Rello J, Mokart D, Lemiale V, Mirouse A (February 2020). "Diagnosis of severe respiratory infections in immunocompromised patients". Intensive Care Medicine. 46 (2): 298–314. doi:10.1007/s00134-019-05906-5. PMC 7080052. PMID 32034433.
- ↑ "Streptococcal Infections - Infectious Diseases". MSD Manual Professional Edition. Archived from the original on 5 July 2021. Retrieved 2 May 2021.
- ↑ Fish DN (February 2002). "Optimal antimicrobial therapy for sepsis". Am J Health Syst Pharm. 59 (Suppl 1): S13–9. doi:10.1093/ajhp/59.suppl_1.S13. PMID 11885408. Archived from the original on 2023-04-24. Retrieved 2023-02-17.
- ↑ Heise E (1982). "Diseases associated with immunosuppression". Environ Health Perspect. 43: 9–19. doi:10.2307/3429162. JSTOR 3429162. PMC 1568899. PMID 7037390.
- ↑ Saiman L (2004). "Microbiology of early CF lung disease". Paediatr Respir Rev. 5 (Suppl A): S367–9. doi:10.1016/S1526-0542(04)90065-6. PMID 14980298.
- ↑ Belland R, Ouellette S, Gieffers J, Byrne G (2004). "Chlamydia pneumoniae and atherosclerosis". Cell Microbiol. 6 (2): 117–27. doi:10.1046/j.1462-5822.2003.00352.x. PMID 14706098. S2CID 45218449.
- ↑ Muzny CA, Schwebke JR (August 2016). "Pathogenesis of Bacterial Vaginosis: Discussion of Current Hypotheses". The Journal of Infectious Diseases. 214 Suppl 1: S1–5. doi:10.1093/infdis/jiw121. PMC 4957507. PMID 27449868.
- ↑ "Urinary Tract Infections". Archived from the original on 2010-02-01. Retrieved 2010-02-04.
- ↑ "Adult Health Advisor 2005.4: Bacteria in Urine, No Symptoms (Asymptomatic Bacteriuria)". Archived from the original on 2007-07-12. Retrieved 2007-08-25.
- ↑ "Impetigo". National Health Service. 19 October 2017. Archived from the original on 15 June 2015. Retrieved 17 February 2023. Page last reviewed: 17/07/2014
- ↑ Kumar, Vinay; Abbas, Abul K.; Fausto, Nelson; & Mitchell, Richard N. (2007). Robbins Basic Pathology (8th ed.). Saunders Elsevier. pp. 843 ISBN 978-1-4160-2973-1
- ↑ "erysipelas" at Dorland's Medical Dictionary
- ↑ "cellulitis" at Dorland's Medical Dictionary
- ↑ Greenwood, David; Barer, Mike; Slack, Richard; Irving, Will (2012). "Bacterial Pathogenicity". Medical Microbiology, a Guide to Microbial Infections: Pathogenesis, Immunity, Laboratory Investigation, and Control (18th ed.). Edinburgh: Churchill Livingstone. pp. 156–167. ISBN 9780702040894.
- ↑ Rudkin JK, McLoughlin RM, Preston A, Massey RC (September 2017). "Bacterial toxins: Offensive, defensive, or something else altogether?". PLOS Pathogens. 13 (9): e1006452. doi:10.1371/journal.ppat.1006452. PMC 5608399. PMID 28934339.
- ↑ Tortora, Gerald J.; Funke, Berdell R.; Case, Christine L. (2016). "Microbial Mechanisms of Pathogenicity". Microbiology, an Introduction (12th ed.). Pearson Education. pp. 417–438. ISBN 978-0-321-92915-0.
- ↑ Nash, Anthony A.; Dalziel, Robert G.; Fitzgerald, J. Ross (2015). "Mechanisms of Cell and Tissue Damage". Mims' Pathogenesis of Infectious Disease (6th ed.). London: Academic Press. pp. 171–231. ISBN 978-0-12-397188-3.
- 1 2 Tortota, Gerard (2013). Microbiology an Introduction. ISBN 978-0-321-73360-3.
- ↑ Hosmer, Jennifer; Nasreen, Marufa; Dhouib, Rabeb; Essilfie, Ama-Tawiah; Schirra, Horst Joachim; Henningham, Anna; Fantino, Emmanuelle; Sly, Peter; McEwan, Alastair G.; Kappler, Ulrike (2022-01-27). "Access to highly specialized growth substrates and production of epithelial immunomodulatory metabolites determine survival of Haemophilus influenzae in human airway epithelial cells". PLOS Pathogens. 18 (1): e1010209. doi:10.1371/journal.ppat.1010209. ISSN 1553-7374. PMC 8794153. PMID 35085362.
- ↑ Cassells AC (2012). "Pathogen and biological contamination management in plant tissue culture: phytopathogens, vitro pathogens, and vitro pests". Plant Cell Culture Protocols. Methods in Molecular Biology. Vol. 877. pp. 57–80. doi:10.1007/978-1-61779-818-4_6. ISBN 978-1-61779-817-7. PMID 22610620.
- ↑ Yonath A, Bashan A (2004). "Ribosomal crystallography: initiation, peptide bond formation, and amino acid polymerization are hampered by antibiotics". Annu Rev Microbiol. 58: 233–51. doi:10.1146/annurev.micro.58.030603.123822. PMID 15487937.
- ↑ Khachatourians GG (November 1998). "Agricultural use of antibiotics and the evolution and transfer of antibiotic-resistant bacteria". CMAJ. 159 (9): 1129–36. PMC 1229782. PMID 9835883.
- ↑ Keen, E. C. (2012). "Phage Therapy: Concept to Cure". Frontiers in Microbiology. 3: 238. doi:10.3389/fmicb.2012.00238. PMC 3400130. PMID 22833738.
- 1 2 3 4 5 6 7 8 9 10 11 12 13 14 15 16 17 18 Unless else specified in boxes then ref is: Fisher, Bruce; Harvey, Richard P.; Champe, Pamela C. (2007). Lippincott's Illustrated Reviews: Microbiology (Lippincott's Illustrated Reviews Series). Hagerstown, MD: Lippincott Williams & Wilkins. pp. 332–353. ISBN 978-0-7817-8215-9.
- ↑ Kurzynski TA, Boehm DM, Rott-Petri JA, Schell RF, Allison PE (1988). "Comparison of modified Bordet-Gengou and modified Regan-Lowe media for the isolation of Bordetella pertussis and Bordetella parapertussis". J. Clin. Microbiol. 26 (12): 2661–3. doi:10.1128/JCM.26.12.2661-2663.1988. PMC 266968. PMID 2906642.
- 1 2 3 4 5 6 7 8 9 10 11 12 13 14 15 16 17 18 19 20 21 22 23 24 25 26 27 28 29 30 31 32 33 34 35 36 37 38 39 40 41 42 43 44 45 46 47 48 49 50 51 52 53 54 55 56 57 58 59 60 61 62 63 64 65 66 67 68 69 70 71 72 73 74 75 76 77 78 79 80 81 82 83 84 85 86 87 88 89 90 91 92 93 94 95 96 97 98 99 100 101 102 103 104 105 106 107 108 109 110 111 112 113 114 115 116 117 118 119 120 121 122 123 124 125 126 127 128 129 130 131 132 133 134 135 136 137 138 139 140 141 142 143 144 145 146 147 148 149 150 151 152 153 154 155 156 157 158 159 160 161 162 163 164 165 166 167 168 169 170 171 172 173 174 175 176 177 178 179 180 181 182 183 184 185 186 187 188 189 190 191 192 193 194 195 196 197 198 199 200 201 202 203 204 205 206 207 208 209 210 211 212 213 214 215 216 217 218 219 220 221 222 223 224 225 226 227 228 229 230 231 232 233 234 235 236 237 238 239 240 241 242 243 244 245 246 247 248 249 250 251 252 253 254 255 256 257 258 259 260 261 262 263 264 265 266 267 268 269 270 271 272 273 274 275 276 277 278 279 280 281 282 283 284 285 286 287 288 289 290 291 292 293 294 295 296 297 298 299 300 301 302 303 304 Fisher, Bruce; Harvey, Richard P.; Champe, Pamela C. (2007). Lippincott's Illustrated Reviews: Microbiology (Lippincott's Illustrated Reviews Series). Hagerstown, MD: Lippincott Williams & Wilkins. pp. 332–353. ISBN 978-0-7817-8215-9.
- 1 2 3 Epps SV, Harvey RB, Hume ME, Phillips TD, Anderson RC, Nisbet DJ (2013). "Foodborne Campylobacter: infections, metabolism, pathogenesis and reservoirs". International Journal of Environmental Research and Public Health. 10 (12): 6292–304. doi:10.3390/ijerph10126292. PMC 3881114. PMID 24287853.
- 1 2 Bowden GHW (1996). Baron S; et al. (eds.). Actinomycosis in: Baron's Medical Microbiology (4th ed.). Univ of Texas Medical Branch. ISBN 978-0-9631172-1-2. (via NCBI Bookshelf).
- ↑ Baron, Samuel (1996). Medical Microbiology (4th ed.). University of Texas Medical Branch at Galveston, Galveston, Texas. ISBN 978-0-9631172-1-2.
- ↑ Rollins, David M. (2000). "BSCI424 Laboratory Media". University of Maryland. Archived from the original on 2009-01-22. Retrieved 2008-11-18.
- ↑ Cain, Donna (January 14, 2015). "MacConkey Agar (CCCCD Microbiology". Collin College. Archived from the original on April 26, 2015. Retrieved May 3, 2015.
- ↑ Gunn BA (1984). "Chocolate agar, a differential medium for gram-positive cocci". Journal of Clinical Microbiology. 20 (4): 822–3. doi:10.1128/JCM.20.4.822-823.1984. PMC 271442. PMID 6490866.
- ↑ Stevenson TH, Castillo A, Lucia LM, Acuff GR (2000). "Growth of Helicobacter pylori in various liquid and plating media". Lett. Appl. Microbiol. 30 (3): 192–6. doi:10.1046/j.1472-765x.2000.00699.x. PMID 10747249. S2CID 24668819.
- ↑ Johnson RC, Harris VG (1967). "Differentiation of Pathogenic and Saprophytic Leptospires I. Growth at Low Temperatures". J. Bacteriol. 94 (1): 27–31. doi:10.1128/JB.94.1.27-31.1967. PMC 251866. PMID 6027998.
- ↑ "Thayer Martin Agar (Modified) Procedure" (PDF). University of Nebraska-Medical Center, Clinical Laboratory Science Program. Archived (PDF) from the original on 2015-06-20. Retrieved 2015-05-03.
- ↑ Allen, Mary E. (2005). "MacConkey Agar Plates Protocols". American Society for Microbiology. Archived from the original on 2015-05-07. Created: 30 September 2005. Last update: 01 April 2013
- ↑ "Hektoen Enteric Agar". Austin Community College District. Archived from the original on 2015-04-29. Retrieved 2015-05-03.
- ↑ Cassell GH, Waites KB, Crouse DT, Rudd PT, Canupp KC, Stagno S, Cutter GR (1988). "Association of Ureaplasma urealyticum infection of the lower respiratory tract with chronic lung disease and death in very-low-birth-weight infants". Lancet. 2 (8605): 240–5. doi:10.1016/s0140-6736(88)92536-6. PMID 2899235. S2CID 6685738.
- ↑ Pfeffer, C.; Oliver, J.D. (2003). "A comparison of thiosulphate-citrate-bile salts-sucrose (TCBS) agar and thiosulphate-chloride-iodide (TCI) agar for the isolation of Vibrio species from estuarine environments". Letters in Applied Microbiology. 36 (3): 150–151. doi:10.1046/j.1472-765X.2003.01280.x. PMID 12581373. S2CID 34004290.
- ↑ "Yersinia pestis" (PDF). Wadsworth Center. 2006. Archived (PDF) from the original on 2015-09-22. Retrieved 2023-02-17.
- 1 2 3 4 5 6 7 8 9 10 11 12 13 14 15 16 17 18 19 20 21 22 23 24 25 26 27 28 29 30 31 32 33 34 35 36 37 38 39 40 41 42 43 44 45 46 47 48 49 50 51 52 53 54 55 56 57 58 59 60 61 62 63 64 65 66 67 68 69 70 71 72 73 74 75 76 77 78 79 80 81 82 83 84 85 86 87 88 89 90 91 92 93 94 95 96 97 98 99 100 101 102 103 104 105 106 107 108 109 110 111 112 113 114 115 116 117 118 119 120 121 122 123 124 125 126 127 128 129 130 131 132 133 134 135 136 137 138 139 140 141 142 143 144 145 146 147 148 149 150 151 152 153 154 155 156 157 158 159 160 161 162 163 164 165 166 167 168 169 170 171 172 173 174 175 176 177 178 179 180 181 182 183 184 185 186 187 188 189 190 191 192 193 194 195 196 197 198 199 200 201 202 203 204 205 206 207 208 209 210 211 212 213 214 215 "Bacteria Table" (PDF). Creighton University School of Medicine. Archived from the original (PDF) on 2015-05-01. Retrieved 2015-05-03.
- ↑ Brook, I (Oct 2008). "Actinomycosis: diagnosis and management". Southern Medical Journal. 101 (10): 1019–23. doi:10.1097/SMJ.0b013e3181864c1f. PMID 18791528. S2CID 19554893.
- ↑ Mabeza, GF; Macfarlane J (March 2003). "Pulmonary actinomycosis". European Respiratory Journal. 21 (3): 545–551. doi:10.1183/09031936.03.00089103. PMID 12662015.
- ↑ "Anthrax in animals". Food and Agriculture Organization. 2001. Archived from the original on 2015-07-03. Retrieved 2023-02-17.
- ↑ "CDC Anthrax Q & A: Treatment". Archived from the original on 5 May 2011. Retrieved 4 April 2011.
- ↑ "FDA approves raxibacumab to treat inhalational anthrax". Food and Drug Administration. Archived from the original on 17 December 2012. Retrieved 14 December 2012.
- 1 2 Itzhak Brook (Jan 28, 2014). "Bacteroides Infection Follow-up". Medscape. Archived from the original on 2015-09-18. Retrieved 2015-09-25.
- ↑ Shapiro ED (2014). "Clinical practice. Lyme disease". The New England Journal of Medicine. 370 (18): 1724–31. doi:10.1056/NEJMcp1314325. PMC 4487875. PMID 24785207.
- 1 2 Sanchez JL (2015). "Clinical Manifestations and Treatment of Lyme Disease". Clinics in Laboratory Medicine. 35 (4): 765–78. doi:10.1016/j.cll.2015.08.004. PMID 26593256.
- ↑ Halperin JJ (2015). "Nervous System Lyme Disease". Clinics in Laboratory Medicine. 35 (4): 779–95. doi:10.1016/j.cll.2015.07.002. PMID 26593257.
- 1 2 3 4 5 6 Barbour, Alan G. (2017). "Relapsing Fever". In Kasper, Dennis L.; Fauci, Anthony S. (eds.). Harrison's Infectious Diseases (3rd ed.). New York: McGraw Hill Education. pp. 678–687. ISBN 978-1-259-83597-1.
- ↑ Cutler SJ (2015). "Relapsing Fever Borreliae: A Global Review". Clinics in Laboratory Medicine. 35 (4): 847–65. doi:10.1016/j.cll.2015.07.001. PMID 26593261.
- ↑ Atkinson, William (May 2012). Tetanus Epidemiology and Prevention of Vaccine-Preventable Diseases (12 ed.). Public Health Foundation. pp. 291–300. ISBN 9780983263135. Archived from the original on 13 February 2015. Retrieved 12 February 2015.
- ↑ "Diphtheria vaccine" (PDF). Wkly Epidemiol Rec. 81 (3): 24–32. 20 January 2006. PMID 16671240. Archived (PDF) from the original on 6 June 2015.
- ↑ "ESCHERICHIA COLI". Public Health Agency of Canada. 2012-04-30. Archived from the original on 2015-05-10. Retrieved 2015-06-02.
- 1 2 "Signs & Symptoms". Centers for Disease Control and Prevention. 13 December 2018. Archived from the original on 8 November 2017. Retrieved 17 February 2023. Page last reviewed: October 26, 2015
- ↑ Ryan, KJ; Ray, CG, eds. (2004). Sherris Medical Microbiology (4th ed.). McGraw Hill. ISBN 978-0-8385-8529-0.
- ↑ "Klebsiella pneumoniae in Healthcare Settings". Centers for Disease Control and Prevention. 19 February 2021. Archived from the original on 19 October 2021. Retrieved 17 February 2023. Page last reviewed: November 24, 2010. Page last updated: August 27, 2012
- ↑ Slack, A (Jul 2010). "Leptospirosis". Australian Family Physician. 39 (7): 495–8. PMID 20628664.
- ↑ McBride, AJ; Athanazio, DA; Reis, MG; Ko, AI (Oct 2005). "Leptospirosis". Current Opinion in Infectious Diseases. 18 (5): 376–86. doi:10.1097/01.qco.0000178824.05715.2c. PMID 16148523. S2CID 220576544.
- 1 2 Hartskeerl, Rudy A.; Wagenaar, Jiri F.P. (2017). "Leptospirosis". In Kasper, Dennis L.; Fauci, Anthony S. (eds.). Harrison's Infectious Diseases. New York: McGraw Hill Education. pp. 672–678. ISBN 978-1-259-83597-1.
- ↑ "Leprosy Fact sheet N°101". World Health Organization. January 2014. Archived from the original on 2013-12-12.
- ↑ "Tuberculosis Fact sheet N°104". WHO. October 2015. Archived from the original on 23 August 2012. Retrieved 11 February 2016.
- ↑ Institut Pasteur Press Office - Vaccine against shigellosis (bacillary dysentery):a promising clinical trial Archived 2009-02-25 at the Wayback Machine 15 January 2009. Retrieved on 27 February 2009
- ↑ Levinson, W. (2010). Review of Medical Microbiology and Immunology (11th ed.). pp. 94–9.
- ↑ "Syphilis - CDC Fact Sheet (Detailed)". CDC. 2 November 2015. Archived from the original on 6 February 2016. Retrieved 3 February 2016.
- ↑ Kent ME, Romanelli F (February 2008). "Reexamining syphilis: an update on epidemiology, clinical manifestations, and management". Annals of Pharmacotherapy. 42 (2): 226–36. doi:10.1345/aph.1K086. PMID 18212261. S2CID 23899851.
- ↑ Hook EW (2017). "Syphilis". Lancet. 389 (10078): 1550–1557. doi:10.1016/S0140-6736(16)32411-4. PMID 27993382. S2CID 208793678.
- ↑ Zhou D, Han Y, Yang R (2006). "Molecular and physiological insights into plague transmission, virulence and etiology". Microbes Infect. 8 (1): 273–84. doi:10.1016/j.micinf.2005.06.006. PMID 16182593.
- ↑ Wagle PM. (1948). "Recent advances in the treatment of bubonic plague". Indian J Med Sci. 2: 489–94.
- ↑ Meyer KF. (1950). "Modern therapy of plague". JAMA. 144 (12): 982–5. doi:10.1001/jama.1950.02920120006003. PMID 14774219.
- ↑ Kilonzo BS, Makundi RH, Mbise TJ (1992). "A decade of plague epidemiology and control in the Western Usambara mountains, north-east Tanzania". Acta Tropica. 50 (4): 323–9. doi:10.1016/0001-706X(92)90067-8. PMID 1356303.
- ↑ Bubeck SS, Dube PH (September 2007). "Yersinia pestis CO92ΔyopH Is a Potent Live, Attenuated Plague Vaccine". Clin. Vaccine Immunol. 14 (9): 1235–8. doi:10.1128/CVI.00137-07. PMC 2043315. PMID 17652523.
- 1 2 Bernstein H, Bernstein C, Michod RE (2018). Sex in microbial pathogens. Infection, Genetics and Evolution volume 57, pages 8-25. https://doi.org/10.1016/j.meegid.2017.10.024 Archived 2023-04-24 at the Wayback Machine
External links
| Classification |
|
|---|
- Bacterial Pathogen Pronunciation Archived 2012-03-08 at the Wayback Machine by Neal R. Chamberlain, Ph.D. at A.T. Still University
- Pathogenic bacteria Archived 2011-07-27 at the Wayback Machine genomes and related information at PATRIC Archived 2011-07-27 at the Wayback Machine, a Bioinformatics Resource Center funded by NIAID Archived 2021-08-26 at the Wayback Machine