Stimulant

Stimulants (also often referred to as psychostimulants or colloquially as uppers) is an overarching term that covers many drugs including those that increase activity of the central nervous system and the body,[1] drugs that are pleasurable and invigorating, or drugs that have sympathomimetic effects.[2] Stimulants are widely used throughout the world as prescription medicines as well as without a prescription (either legally or illicitly) as performance-enhancing or recreational drugs. Among narcotics, stimulants produce a noticeable crash or comedown at the end of their effects. The most frequently prescribed stimulants as of 2013 were lisdexamfetamine, methylphenidate (Ritalin), and amphetamine.[3] It was estimated in 2015 that the percentage of the world population that had used cocaine during a year was 0.4%. For the category "amphetamines and prescription stimulants" (with "amphetamines" including amphetamine and methamphetamine) the value was 0.7%, and for Ecstasy 0.4%.[4]
Effects
Acute
Stimulants in therapeutic doses, such as those given to patients with ADHD, increases ability to focus, vigor, sociability, libido and may elevate mood. However, in higher doses stimulants may actually decrease the ability to focus, a principle of the Yerkes-Dodson Law. In higher doses stimulants may also produce euphoria, vigor, and decrease need for sleep. Many, but not all, stimulants have ergogenic effects. Drugs such as ephedrine, pseudoephedrine, amphetamine and methylphenidate have well documented ergogenic effects, while cocaine has the opposite effect.[5] Neurocognitive enhancing effects of stimulants, specifically modafinil, amphetamine and methylphenidate have been documented in healthy adolescents, and is a commonly cited reason among illicit drug users for use, particularly among college students in the context of studying.[6]
In some cases psychiatric phenomenon may emerge such as stimulant psychosis, paranoia, and suicidal ideation. Acute toxicity has been reportedly associated with a homicide, paranoia, aggressive behavior, motor dysfunction, and punding. The violent and aggressive behavior associated with acute stimulant toxicity may partially be driven by paranoia.[7] Most drugs classified as stimulants are sympathomimetics, that is they stimulate the sympathetic branch of the autonomic nervous system. This leads to effects such as mydriasis, increased heart rate, blood pressure, respiratory rate and body temperature.[8] When these changes become pathological, they are called arrhythmia, hypertension, and hyperthermia, and may lead to rhabdomyolysis, stroke, cardiac arrest, or seizures. However, given the complexity of the mechanisms that underlie these potentially fatal outcomes of acute stimulant toxicity, it is impossible to determine what dose may be lethal.[9]
Chronic
Assessment of the effects of stimulants is relevant given the large population currently taking stimulants. A systematic review of cardiovascular effects of prescription stimulants found no association in children, but found a correlation between prescription stimulant use and ischemic heart attacks.[10] A review over a four-year period found that there were few negative effects of stimulant treatment, but stressed the need for longer-term studies.[11] A review of a year long period of prescription stimulant use in those with ADHD found that cardiovascular side effects were limited to transient increases in blood pressure only.[12] Initiation of stimulant treatment in those with ADHD in early childhood appears to carry benefits into adulthood with regard to social and cognitive functioning, and appears to be relatively safe.[13]
Abuse of prescription stimulants (not following physician instruction) or of illicit stimulants carries many negative health risks. Abuse of cocaine, depending upon route of administration, increases risk of cardiorespiratory disease, stroke, and sepsis.[14] Some effects are dependent upon the route of administration, with intravenous use associated with the transmission of many disease such as Hepatitis C, HIV/AIDS and potential medical emergencies such as infection, thrombosis or pseudoaneurysm,[15] while inhalation may be associated with increased lower respiratory tract infection, lung cancer, and pathological restricting of lung tissue.[16] Cocaine may also increase risk for autoimmune disease[17][18][19] and damage nasal cartilage. Abuse of methamphetamine produces similar effects as well as marked degeneration of dopaminergic neurons, resulting in an increased risk for Parkinson's disease.[20][21][22][23]
Medical uses
Stimulants have been used in medicine for many conditions including obesity, sleep disorders, mood disorders, impulse control disorders, asthma, nasal congestion and, in case of cocaine, as local anesthetics.[24] Drugs used to treat obesity are called anorectics and generally include drugs that follow the general definition of a stimulant, but other drugs such as cannabinoid receptor antagonists also belong to this group.[25][26] Eugeroics are used in management of sleep disorders characterized by excessive daytime sleepiness, such as narcolepsy, and include stimulants such as modafinil.[27][28] Stimulants are used in impulse control disorders such as ADHD[29] and off-label in mood disorders such as major depressive disorder to increase energy, focus and elevate mood.[30] Stimulants such as epinephrine,[31] theophylline and salbutamol[32] orally have been used to treat asthma, but inhaled adrenergic drugs are now preferred due to less systemic side effects. Pseudoephedrine is used to relieve nasal or sinus congestion caused by the common cold, sinusitis, hay fever and other respiratory allergies; it is also used to relieve ear congestion caused by ear inflammation or infection.[33][34]
Chemistry
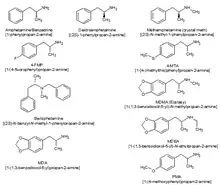
Classifying stimulants is difficult, because of the large number of classes the drugs occupy, and the fact that they may belong to multiple classes; for example, ecstasy can be classified as a substituted methylenedioxyphenethylamine, a substituted amphetamine and consequently, a substituted phenethylamine.
When referring to stimulants, the parent drug (e.g., amphetamine) will always be expressed in the singular; with the word "substituted" placed before the parent drug (substituted amphetamines).
Major stimulant classes include phenethylamines and their daughter class substituted amphetamines.
Amphetamines (class)
Substituted amphetamines are a class of compounds based upon the amphetamine structure;[35] it includes all derivative compounds which are formed by replacing, or substituting, one or more hydrogen atoms in the amphetamine core structure with substituents.[35][36][37] Examples of substituted amphetamines are amphetamine (itself),[35][36] methamphetamine,[35] ephedrine,[35] cathinone,[35] phentermine,[35] mephentermine,[35] bupropion,[35] methoxyphenamine,[35] selegiline,[35] amfepramone,[35] pyrovalerone,[35] MDMA (ecstasy), and DOM (STP). Many drugs in this class work primarily by activating trace amine-associated receptor 1 (TAAR1);[38] in turn, this causes reuptake inhibition and effluxion, or release, of dopamine, norepinephrine, and serotonin.[38] An additional mechanism of some substituted amphetamines is the release of vesicular stores of monoamine neurotransmitters through VMAT2, thereby increasing the concentration of these neurotransmitters in the cytosol, or intracellular fluid, of the presynaptic neuron.[39]
Amphetamines-type stimulants are often used for their therapeutic effects. Physicians sometimes prescribe amphetamine to treat major depression, where subjects do not respond well to traditional SSRI medications, but evidence supporting this use is poor/mixed.[40] Notably, two recent large phase III studies of lisdexamfetamine (a prodrug to amphetamine) as an adjunct to an SSRI or SNRI in the treatment of major depressive disorder showed no further benefit relative to placebo in effectiveness.[41] Numerous studies have demonstrated the effectiveness of drugs such as Adderall (a mixture of salts of amphetamine and dextroamphetamine) in controlling symptoms associated with ADHD. Due to their availability and fast-acting effects, substituted amphetamines are prime candidates for abuse.[42]
Cocaine analogues
Hundreds of cocaine analogues have been created, all of them usually maintaining a benzyloxy connected to the 3 carbon of a tropane. Various modifications include substitutions on the benzene ring, as well as additions or substitutions in place of the normal carboxylate on the tropane 2 carbon. Various compound with similar structure activity relationships to cocaine that aren't technically analogues have been developed as well.
Mechanisms of action
Most stimulants exert their activating effects by enhancing catecholamine neurotransmission. Catecholamine neurotransmitters are employed in regulatory pathways implicated in attention, arousal, motivation, task salience and reward anticipation. Classical stimulants either block the reuptake or stimulate the efflux of these catecholamines, resulting in increased activity of their circuits. Some stimulants, specifically those with empathogenic and hallucinogenic effects, also affect serotonergic transmission. Some stimulants, such as some amphetamine derivatives and, notably, yohimbine, can decrease negative feedback by antagonizing regulatory autoreceptors.[43] Adrenergic agonists, such as, in part, ephedrine, act by directly binding to and activating adrenergic receptors, producing sympathomimetic effects.
There are also more indirect mechanisms a drug can elicit activating effects. Caffeine is an adenosine receptor antagonist, and only indirectly increases catecholamine transmission in the brain.[44] Pitolisant is an H3-receptor inverse agonist. As H3 receptors mainly act as autoreceptors, pitolisant decreases negative feedback to histaminergic neurons, enhancing histaminergic transmission.
Notable stimulants
Amphetamine
Amphetamine is a potent central nervous system (CNS) stimulant of the phenethylamine class that is approved for the treatment of attention deficit hyperactivity disorder (ADHD) and narcolepsy.[45] Amphetamine is also used off-label as a performance and cognitive enhancer, and recreationally as an aphrodisiac and euphoriant.[46][47][48][49] Although it is a prescription medication in many countries, unauthorized possession and distribution of amphetamine is often tightly controlled due to the significant health risks associated with uncontrolled or heavy use.[50][51] As a consequence, amphetamine is illegally manufactured in clandestine labs to be trafficked and sold to users.[52] Based upon drug and drug precursor seizures worldwide, illicit amphetamine production and trafficking is much less prevalent than that of methamphetamine.[52]
The first pharmaceutical amphetamine was Benzedrine, a brand of inhalers used to treat a variety of conditions.[53][54] Because the dextrorotary isomer has greater stimulant properties, Benzedrine was gradually discontinued in favor of formulations containing all or mostly dextroamphetamine. Presently, it is typically prescribed as mixed amphetamine salts, dextroamphetamine, and lisdexamfetamine.[53][55]
Amphetamine is a norepinephrine-dopamine releasing agent (NDRA). It enters neurons through dopamine and norepinephrine transporters and facilitates neurotransmitter efflux by activating TAAR1 and inhibiting VMAT2.[38] At therapeutic doses, this causes emotional and cognitive effects such as euphoria, change in libido, increased arousal, and improved cognitive control.[47][48][56] Likewise, it induces physical effects such as decreased reaction time, fatigue resistance, and increased muscle strength.[46] In contrast, supratherapeutic doses of amphetamine are likely to impair cognitive function and induce rapid muscle breakdown.[45][47][57] Very high doses can result in psychosis (e.g., delusions and paranoia), which very rarely occurs at therapeutic doses even during long-term use.[58][59] As recreational doses are generally much larger than prescribed therapeutic doses, recreational use carries a far greater risk of serious side effects, such as dependence, which only rarely arises with therapeutic amphetamine use.[45][57][58]
Caffeine

Caffeine is a stimulant compound belonging to the xanthine class of chemicals naturally found in coffee, tea, and (to a lesser degree) cocoa or chocolate. It is included in many soft drinks, as well as a larger amount in energy drinks. Caffeine is the world's most widely used psychoactive drug and by far the most common stimulant. In North America, 90% of adults consume caffeine daily.[60] A few jurisdictions restrict its sale and use. Caffeine is also included in some medications, usually for the purpose of enhancing the effect of the primary ingredient, or reducing one of its side-effects (especially drowsiness). Tablets containing standardized doses of caffeine are also widely available.
Caffeine's mechanism of action differs from many stimulants, as it produces stimulant effects by inhibiting adenosine receptors.[61] Adenosine receptors are thought to be a large driver of drowsiness and sleep, and their action increases with extended wakefulness.[62] Caffeine has been found to increase striatal dopamine in animal models,[63] as well as inhibit the inhibitory effect of adenosine receptors on dopamine receptors,[64] however the implications for humans are unknown. Unlike most stimulants, caffeine has no addictive potential. Caffeine does not appear to be a reinforcing stimulus, and some degree of aversion may actually occur, which people preferring placebo over caffeine in a study on drug abuse liability published in an NIDA research monograph.[65] In large telephone surveys only 11% reported dependence symptoms. However, when people were tested in labs, only half of those who claim dependence actually experienced it, casting doubt on caffeine's ability to produce dependence and putting societal pressures in the spotlight.[66]
Coffee consumption is associated with a lower overall risk of cancer.[67] This is primarily due to a decrease in the risks of hepatocellular and endometrial cancer, but it may also have a modest effect on colorectal cancer.[68] There does not appear to be a significant protective effect against other types of cancers, and heavy coffee consumption may increase the risk of bladder cancer.[68] A protective effect of caffeine against Alzheimer's disease is possible, but the evidence is inconclusive.[69][70][71] Moderate coffee consumption may decrease the risk of cardiovascular disease,[72] and it may somewhat reduce the risk of type 2 diabetes.[73] Drinking 1-3 cups of coffee per day does not affect the risk of hypertension compared to drinking little or no coffee. However those who drink 2–4 cups per day may be at a slightly increased risk.[74] Caffeine increases intraocular pressure in those with glaucoma but does not appear to affect normal individuals.[75] It may protect people from liver cirrhosis.[76] There is no evidence that coffee stunts a child's growth.[77] Caffeine may increase the effectiveness of some medications including ones used to treat headaches.[78] Caffeine may lessen the severity of acute mountain sickness if taken a few hours prior to attaining a high altitude.[79]
Ephedrine
Ephedrine is a sympathomimetic amine similar in molecular structure to the well-known drugs phenylpropanolamine and methamphetamine, as well as to the important neurotransmitter epinephrine (adrenaline). Ephedrine is commonly used as a stimulant, appetite suppressant, concentration aid, and decongestant, and to treat hypotension associated with anaesthesia.
In chemical terms, it is an alkaloid with a phenethylamine skeleton found in various plants in the genus Ephedra (family Ephedraceae). It works mainly by increasing the activity of norepinephrine (noradrenaline) on adrenergic receptors.[80] It is most usually marketed as the hydrochloride or sulfate salt.
The herb má huáng (Ephedra sinica), used in traditional Chinese medicine (TCM), contains ephedrine and pseudoephedrine as its principal active constituents. The same may be true of other herbal products containing extracts from other Ephedra species.
MDMA
3,4-Methylenedioxymethamphetamine (MDMA, ecstasy, or molly) is a euphoriant, empathogen, and stimulant of the amphetamine class.[81] Briefly used by some psychotherapists as an adjunct to therapy, the drug became popular recreationally and the DEA listed MDMA as a Schedule I controlled substance, prohibiting most medical studies and applications. MDMA is known for its entactogenic properties. The stimulant effects of MDMA include hypertension, anorexia (appetite loss), euphoria, social disinhibition, insomnia (enhanced wakefulness/inability to sleep), improved energy, increased arousal, and increased perspiration, among others. Relative to catecholaminergic transmission, MDMA enhances serotonergic transmission significantly more, when compared to classical stimulants like amphetamine. MDMA does not appear to be significantly addictive or dependence forming.[82]
Due to the relative safety of MDMA, some researchers such as David Nutt have criticized the scheduling level, writing a satirical article finding MDMA to be 28 times less dangerous than horseriding, a condition he termed "equasy" or "Equine Addiction Syndrome".[83]
MDPV
Methylenedioxypyrovalerone (MDPV) is a psychoactive drug with stimulant properties that acts as a norepinephrine-dopamine reuptake inhibitor (NDRI).[84] It was first developed in the 1960s by a team at Boehringer Ingelheim.[85] MDPV remained an obscure stimulant until around 2004, when it was reported to be sold as a designer drug. Products labeled as bath salts containing MDPV were previously sold as recreational drugs in gas stations and convenience stores in the United States, similar to the marketing for Spice and K2 as incense.[86][87]
Incidents of psychological and physical harm have been attributed to MDPV use.[88][89]
Mephedrone
Mephedrone is a synthetic stimulant drug of the amphetamine and cathinone classes. Slang names include drone[90] and MCAT.[91] It is reported to be manufactured in China and is chemically similar to the cathinone compounds found in the khat plant of eastern Africa. It comes in the form of tablets or a powder, which users can swallow, snort, or inject, producing similar effects to MDMA, amphetamines, and cocaine.
Mephedrone was first synthesized in 1929, but did not become widely known until it was rediscovered in 2003. By 2007, mephedrone was reported to be available for sale on the Internet; by 2008 law enforcement agencies had become aware of the compound; and, by 2010, it had been reported in most of Europe, becoming particularly prevalent in the United Kingdom. Mephedrone was first made illegal in Israel in 2008, followed by Sweden later that year. In 2010, it was made illegal in many European countries, and, in December 2010, the EU ruled it illegal. In Australia, New Zealand, and the US, it is considered an analog of other illegal drugs and can be controlled by laws similar to the Federal Analog Act. In September 2011, the USA temporarily classified mephedrone as illegal, in effect from October 2011.
Methamphetamine
Methamphetamine (contracted from N-methyl-alpha-methylphenethylamine) is a potent psychostimulant of the phenethylamine and amphetamine classes that is used to treat attention deficit hyperactivity disorder (ADHD) and obesity.[92][93][94] Methamphetamine exists as two enantiomers, dextrorotary and levorotary.[95][96] Dextromethamphetamine is a stronger CNS stimulant than levomethamphetamine;[57][95][96] however, both are addictive and produce the same toxicity symptoms at high doses.[96] Although rarely prescribed due to the potential risks, methamphetamine hydrochloride is approved by the United States Food and Drug Administration (USFDA) under the trade name Desoxyn.[93] Recreationally, methamphetamine is used to increase sexual desire, lift the mood, and increase energy, allowing some users to engage in sexual activity continuously for several days straight.[93][97]
Methamphetamine may be sold illicitly, either as pure dextromethamphetamine or in an equal parts mixture of the right- and left-handed molecules (i.e., 50% levomethamphetamine and 50% dextromethamphetamine).[97] Both dextromethamphetamine and racemic methamphetamine are schedule II controlled substances in the United States.[93] Also, the production, distribution, sale, and possession of methamphetamine is restricted or illegal in many other countries due to its placement in schedule II of the United Nations Convention on Psychotropic Substances treaty.[98][99] In contrast, levomethamphetamine is an over-the-counter drug in the United States.[note 1]
In low doses, methamphetamine can cause an elevated mood and increase alertness, concentration, and energy in fatigued individuals.[57][93] At higher doses, it can induce psychosis, rhabdomyolysis, and cerebral hemorrhage.[57][93] Methamphetamine is known to have a high potential for abuse and addiction.[57][93] Recreational use of methamphetamine may result in psychosis or lead to post-withdrawal syndrome, a withdrawal syndrome that can persist for months beyond the typical withdrawal period.[102] Unlike amphetamine and cocaine, methamphetamine is neurotoxic to humans, damaging both dopamine and serotonin neurons in the central nervous system (CNS).[92][94] Unlike the long-term use of amphetamine in prescription doses, which may improve certain brain regions in individuals with ADHD, there is evidence that methamphetamine causes brain damage from long-term use in humans;[92][94] this damage includes adverse changes in brain structure and function, such as reductions in gray matter volume in several brain regions and adverse changes in markers of metabolic integrity.[103][104][94] However, recreational amphetamine doses may also be neurotoxic. [105]
Methylphenidate
Methylphenidate is a stimulant drug that is often used in the treatment of ADHD and narcolepsy and occasionally to treat obesity in combination with diet restraints and exercise. Its effects at therapeutic doses include increased focus, increased alertness, decreased appetite, decreased need for sleep and decreased impulsivity. Methylphenidate is not usually used recreationally, but when it is used, its effects are very similar to those of amphetamines.
Methylphenidate acts as a norepinephrine-dopamine reuptake inhibitor, by blocking the norepinephrine transporter (NET) and the dopamine transporter (DAT). Methylphenidate has a higher affinity for the dopamine transporter than for the norepinephrine transporter, and so its effects are mainly due to elevated dopamine levels caused by the inhibited reuptake of dopamine, however increased norepinephrine levels also contribute to various of the effects caused by the drug.
Methylphenidate is sold under a number of brand names including Ritalin. Other versions include the long lasting tablet Concerta and the long lasting transdermal patch Daytrana.
Cocaine

Cocaine is an SNDRI. Cocaine is made from the leaves of the coca shrub, which grows in the mountain regions of South American countries such as Bolivia, Colombia, and Peru, regions in which it was cultivated and used for centuries mainly by the Aymara people. In Europe, North America, and some parts of Asia, the most common form of cocaine is a white crystalline powder. Cocaine is a stimulant but is not normally prescribed therapeutically for its stimulant properties, although it sees clinical use as a local anesthetic, in particular in ophthalmology.[106] Most cocaine use is recreational and its abuse potential is high (higher than amphetamine), and so its sale and possession are strictly controlled in most jurisdictions. Other tropane derivative drugs related to cocaine are also known such as troparil and lometopane but have not been widely sold or used recreationally.[107]
Nicotine
Nicotine is the active chemical constituent in tobacco, which is available in many forms, including cigarettes, cigars, chewing tobacco, and smoking cessation aids such as nicotine patches, nicotine gum, and electronic cigarettes. Nicotine is used widely throughout the world for its stimulating and relaxing effects. Nicotine exerts its effects through the agonism of nicotinic acetylcholine receptor, resulting in multiple downstream effects such as increase in activity of dopaminergic neurons in the midbrain reward system, and acetaldehyde one of the tobacco constituent decreased the expression of monoamine oxidase in the brain.[108] Nicotine is addictive and dependence forming. Tobacco, the most common source of nicotine, has an overall harm to user and self score 3 percent below cocaine, and 13 percent above amphetamines, ranking 6th most harmful of the 20 drugs assessed, as determined by a multi-criteria decision analysis.[109]
Phenylpropanolamine
Phenylpropanolamine (PPA; Accutrim; β-hydroxyamphetamine), also known as the stereoisomers norephedrine and norpseudoephedrine, is a psychoactive drug of the phenethylamine and amphetamine chemical classes that is used as a stimulant, decongestant, and anorectic agent.[110] It is commonly used in prescription and over-the-counter cough and cold preparations. In veterinary medicine, it is used to control urinary incontinence in dogs under trade names Propalin and Proin.
In the United States, PPA is no longer sold without a prescription due to a proposed increased risk of stroke in younger women. In a few countries in Europe, however, it is still available either by prescription or sometimes over-the-counter. In Canada, it was withdrawn from the market on 31 May 2001.[111] In India, human use of PPA and its formulations were banned on 10 February 2011.[112]
Propylhexedrine
Propylhexedrine (Hexahydromethamphetamine, Obesin) is a stimulant medication, sold over-the-counter in the United States as the cold medication Benzedrex.[113] The drug has also been used as an appetite suppressant in Europe. Propylhexedrine is not an amphetamine, though it is structurally similar; it is instead a cycloalkylamine, and thus has stimulant effects that are less potent than similarly structured amphetamines, such as methamphetamine.
The abuse potential of propylhexedrine is fairly limited, due its limited routes of administration: in the United States, Benzedrex is only available as an inhalant, mixed with lavender oil and menthol. These ingredients cause unpleasant tastes, and abusers of the drug have reported unpleasant "menthol burps". Injection of the drug has been found to cause transient diplopia and brain stem dysfunction.[114][115][116]
Pseudoephedrine
Pseudoephedrine is a sympathomimetic drug of the phenethylamine and amphetamine chemical classes. It may be used as a nasal/sinus decongestant, as a stimulant,[117] or as a wakefulness-promoting agent.[118]
The salts pseudoephedrine hydrochloride and pseudoephedrine sulfate are found in many over-the-counter preparations, either as a single ingredient or (more commonly) in combination with antihistamines, guaifenesin, dextromethorphan, and/or paracetamol (acetaminophen) or another NSAID (such as aspirin or ibuprofen). It is also used as a precursor chemical in the illegal production of methamphetamine.
Catha edulis (Khat)

Khat is a flowering plant native to the Horn of Africa and the Arabian Peninsula.[119][120]
Khat contains a monoamine alkaloid called cathinone, a "keto-amphetamine", that is said to cause excitement, loss of appetite, and euphoria. In 1980, the World Health Organization (WHO) classified it as a drug of abuse that can produce mild to moderate psychological dependence (less than tobacco or alcohol),[121] although the WHO does not consider khat to be seriously addictive.[120] It is banned in some countries, such as the United States, Canada, and Germany, while its production, sale, and consumption are legal in other nations, including Djibouti, Ethiopia, Somalia, and Yemen.[122]
Modafinil
Modafinil, sold under the brand name Provigil among others, is a CNS stimulant used to treat sleepiness due to narcolepsy, shift work sleep disorder, or obstructive sleep apnea. While it has seen off-label use as a purported cognitive enhancer, the research on its effectiveness for this use is not conclusive.
Recreational use and issues of abuse
Stimulants enhance the activity of the central and peripheral nervous systems. Common effects may include increased alertness, awareness, wakefulness, endurance, productivity, and motivation, arousal, locomotion, heart rate, and blood pressure, and a diminished desire for food and sleep. Use of stimulants may cause the body to reduce significantly its production of natural body chemicals that fulfill similar functions. Until the body reestablishes its normal state, once the effect of the ingested stimulant has worn off the user may feel depressed, lethargic, confused, and miserable. This is referred to as a "crash", and may provoke reuse of the stimulant.
Abuse of central nervous system (CNS) stimulants is common. Addiction to some CNS stimulants can quickly lead to medical, psychiatric, and psychosocial deterioration. Drug tolerance, dependence, and sensitization as well as a withdrawal syndrome can occur.[123] Stimulants may be screened for in animal discrimination and self-administration models which have high sensitivity albeit low specificity.[124] Research on a progressive ratio Self-administration protocol has found amphetamine, methylphenidate, modafinil, cocaine, and nicotine to all have a higher break point than placebo that scales with dose indicating reinforcing effects.[125]
| Dependence potentials of common stimulants[121] | ||||
|---|---|---|---|---|
| Drug | Mean | Pleasure | Psychological dependence | Physical dependence |
| Cocaine | 2.39 | 3.0 | 2.8 | 1.3 |
| Tobacco | 2.21 | 2.3 | 2.6 | 1.8 |
| Amphetamine | 1.67 | 2.0 | 1.9 | 1.1 |
| Ecstasy | 1.13 | 1.5 | 1.2 | 0.7 |
Treatment for misuse
Psychosocial treatments, such as contingency management, have demonstrated improved effectiveness when added to treatment as usual consisting of counselling and/or case-management. This is demonstrated with a decrease in dropout rates and a lengthening of periods of abstinence.[126]
Testing
The presence of stimulants in the body may be tested by a variety of procedures. Serum and urine are the common sources of testing material although saliva is sometimes used. Commonly used tests include chromatography, immunologic assay, and mass spectrometry.[127]
See also
Notes
References
- ↑ "stimulant – definition of stimulant in English | Oxford Dictionaries". Oxford Dictionaries | English. Archived from the original on 26 February 2017.
- ↑ Treatment, Center for Substance Abuse (1999). Chapter 2—How Stimulants Affect the Brain and Behavior. Substance Abuse and Mental Health Services Administration (US). Archived from the original on 19 February 2017.
- ↑ "Top 100 Drugs for Q4 2013 by Sales – U.S. Pharmaceutical Statistics". www.drugs.com. Archived from the original on 14 August 2013.
- ↑ "World Drug Report 2015" (PDF). p. 149. Archived (PDF) from the original on 15 February 2016.
- ↑ Avois, L; Robinson, N; Saudan, C; Baume, N; Mangin, P; Saugy, M (7 January 2017). "Central nervous system stimulants and sport practice". British Journal of Sports Medicine. 40 (Suppl 1): i16–i20. doi:10.1136/bjsm.2006.027557. ISSN 0306-3674. PMC 2657493. PMID 16799095.
- ↑ Bagot, Kara Simone; Kaminer, Yifrah (1 April 2014). "Efficacy of stimulants for cognitive enhancement in non-attention deficit hyperactivity disorder youth: a systematic review". Addiction. 109 (4): 547–557. doi:10.1111/add.12460. ISSN 1360-0443. PMC 4471173. PMID 24749160.
- ↑ Morton, W. Alexander; Stockton, Gwendolyn G. (8 January 2017). "Methylphenidate Abuse and Psychiatric Side Effects". Primary Care Companion to the Journal of Clinical Psychiatry. 2 (5): 159–164. doi:10.4088/PCC.v02n0502. ISSN 1523-5998. PMC 181133. PMID 15014637.
- ↑ Treatment, Center for Substance Abuse (1 January 1999). "Chapter 2—How Stimulants Affect the Brain and Behavior". Substance Abuse and Mental Health Services Administration (US). Archived from the original on 19 February 2017.
{{cite journal}}: Cite journal requires|journal=(help) - ↑ Treatment for Stimulant Use Disorders.Chapter 5—Medical Aspects of Stimulant Use Disorders. Center for Substance Abuse Treatment. Treatment for Stimulant Use Disorders. Rockville (MD): Substance Abuse and Mental Health Services Administration (US). 1999. Archived from the original on 19 February 2017.
- ↑ Westover, Arthur N.; Halm, Ethan A. (9 June 2012). "Do prescription stimulants increase the risk of adverse cardiovascular events?: A systematic review". BMC Cardiovascular Disorders. 12: 41. doi:10.1186/1471-2261-12-41. ISSN 1471-2261. PMC 3405448. PMID 22682429.
- ↑ Fredriksen, Mats; Halmøy, Anne; Faraone, Stephen V.; Haavik, Jan (1 June 2013). "Long-term efficacy and safety of treatment with stimulants and atomoxetine in adult ADHD: a review of controlled and naturalistic studies". European Neuropsychopharmacology. 23 (6): 508–527. doi:10.1016/j.euroneuro.2012.07.016. hdl:10852/40257. ISSN 1873-7862. PMID 22917983. S2CID 20400392.
- ↑ Hammerness, Paul G.; Karampahtsis, Chris; Babalola, Ronke; Alexander, Mark E. (1 April 2015). "Attention-deficit/hyperactivity disorder treatment: what are the long-term cardiovascular risks?". Expert Opinion on Drug Safety. 14 (4): 543–551. doi:10.1517/14740338.2015.1011620. ISSN 1744-764X. PMID 25648243. S2CID 39425997.
- ↑ Hechtman, Lily; Greenfield, Brian (1 January 2003). "Long-term use of stimulants in children with attention deficit hyperactivity disorder: safety, efficacy, and long-term outcome". Paediatric Drugs. 5 (12): 787–794. doi:10.2165/00148581-200305120-00002. ISSN 1174-5878. PMID 14658920. S2CID 68191253.
- ↑ Sordo, L; Indave, BI; Barrio, G; Degenhardt, L; de la Fuente, L; Bravo, MJ (1 September 2014). "Cocaine use and risk of stroke: a systematic review". Drug and Alcohol Dependence. 142: 1–13. doi:10.1016/j.drugalcdep.2014.06.041. PMID 25066468.
- ↑ COUGHLIN, P; MAVOR, A (1 October 2006). "Arterial Consequences of Recreational Drug Use". European Journal of Vascular and Endovascular Surgery. 32 (4): 389–396. doi:10.1016/j.ejvs.2006.03.003. PMID 16682239.
- ↑ Tashkin, D. P. (1 March 2001). "Airway effects of marijuana, cocaine, and other inhaled illicit agents". Current Opinion in Pulmonary Medicine. 7 (2): 43–61. doi:10.1097/00063198-200103000-00001. ISSN 1070-5287. PMID 11224724. S2CID 23421796.
- ↑ Trozak D, Gould W (1984). "Cocaine abuse and connective tissue disease". J Am Acad Dermatol. 10 (3): 525. doi:10.1016/S0190-9622(84)80112-7. PMID 6725666.
- ↑ Ramón Peces; Navascués, RA; Baltar, J; Seco, M; Alvarez, J (1999). "Antiglomerular Basement Membrane Antibody-Mediated Glomerulonephritis after Intranasal Cocaine Use". Nephron. 81 (4): 434–438. doi:10.1159/000045328. PMID 10095180. S2CID 26921706.
- ↑ Moore PM, Richardson B (1998). "Neurology of the vasculitides and connective tissue diseases". J. Neurol. Neurosurg. Psychiatry. 65 (1): 10–22. doi:10.1136/jnnp.65.1.10. PMC 2170162. PMID 9667555.
- ↑ Carvalho M, Carmo H, Costa VM, Capela JP, Pontes H, Remião F, Carvalho F, Bastos Mde L (August 2012). "Toxicity of amphetamines: an update". Arch. Toxicol. 86 (8): 1167–1231. doi:10.1007/s00204-012-0815-5. PMID 22392347. S2CID 2873101.
- ↑ Thrash B, Thiruchelvan K, Ahuja M, Suppiramaniam V, Dhanasekaran M (2009). "Methamphetamine-induced neurotoxicity: the road to Parkinson's disease" (PDF). Pharmacol Rep. 61 (6): 966–977. doi:10.1016/s1734-1140(09)70158-6. PMID 20081231. Archived (PDF) from the original on 16 July 2011.
- ↑ Sulzer D, Zecca L (February 2000). "Intraneuronal dopamine-quinone synthesis: a review". Neurotox. Res. 1 (3): 181–195. doi:10.1007/BF03033289. PMID 12835101. S2CID 21892355.
- ↑ Miyazaki I, Asanuma M (June 2008). "Dopaminergic neuron-specific oxidative stress caused by dopamine itself". Acta Med. Okayama. 62 (3): 141–150. doi:10.18926/AMO/30942. PMID 18596830.
- ↑ Harper, S. J.; Jones, N. S. (1 October 2006). "Cocaine: what role does it have in current ENT practice? A review of the current literature". The Journal of Laryngology and Otology. 120 (10): 808–811. doi:10.1017/S0022215106001459. ISSN 1748-5460. PMID 16848922. S2CID 28169472.
- ↑ Kaplan, Lee M. (1 March 2005). "Pharmacological therapies for obesity". Gastroenterology Clinics of North America. 34 (1): 91–104. doi:10.1016/j.gtc.2004.12.002. ISSN 0889-8553. PMID 15823441.
- ↑ Palamara, Kerri L.; Mogul, Harriette R.; Peterson, Stephen J.; Frishman, William H. (1 October 2016). "Obesity: new perspectives and pharmacotherapies". Cardiology in Review. 14 (5): 238–258. doi:10.1097/01.crd.0000233903.57946.fd. ISSN 1538-4683. PMID 16924165.
- ↑ "The Voice of the Patient A series of reports from the U.S. Food and Drug Administration's (FDA's) Patient-Focused Drug Development Initiative" (PDF). Center for Drug Evaluation and Research (CDER) U.S. Food and Drug Administration (FDA). Archived (PDF) from the original on 5 May 2017.
- ↑ Heal, David J; Smith, Sharon L; Gosden, Jane; Nutt, David J (7 January 2017). "Amphetamine, past and present – a pharmacological and clinical perspective". Journal of Psychopharmacology. 27 (6): 479–496. doi:10.1177/0269881113482532. ISSN 0269-8811. PMC 3666194. PMID 23539642.
- ↑ Research, Center for Drug Evaluation and (26 June 2019). "Drug Safety and Availability - FDA Drug Safety Communication: Safety Review Update of Medications used to treat Attention-Deficit/Hyperactivity Disorder (ADHD) in adults". www.fda.gov. Archived from the original on 30 October 2013.
- ↑ Stotz, Gabriele; Woggon, Brigitte; Angst, Jules (1 December 1999). "Psychostimulants in the therapy of treatment-resistant depression Review of the literature and findings from a retrospective study in 65 depressed patients". Dialogues in Clinical Neuroscience. 1 (3): 165–174. ISSN 1294-8322. PMC 3181580. PMID 22034135.
- ↑ Doig RL (February 1905). "Epinephrin; especially in asthma". California State Journal of Medicine. 3 (2): 54–5. PMC 1650334. PMID 18733372.
- ↑ Chu, Eric K.; Drazen, Jeffrey M. (1 June 2005). "Asthma". American Journal of Respiratory and Critical Care Medicine. 171 (11): 1202–1208. doi:10.1164/rccm.200502-257OE. ISSN 1073-449X. PMID 15778490.
- ↑ Bicopoulos D, editor. AusDI: Drug information for the healthcare professional, 2nd edition. Castle Hill: Pharmaceutical Care Information Services; 2002.
- ↑ "Pseudoephedrine (By mouth) – National Library of Medicine". PubMed Health. Archived from the original on 14 February 2014.
- 1 2 3 4 5 6 7 8 9 10 11 12 13 Hagel JM, Krizevski R, Marsolais F, Lewinsohn E, Facchini PJ (2012). "Biosynthesis of amphetamine analogs in plants". Trends Plant Sci. 17 (7): 404–412. doi:10.1016/j.tplants.2012.03.004. PMID 22502775.
Substituted amphetamines, which are also called phenylpropylamino alkaloids, are a diverse group of nitrogen-containing compounds that feature a phenethylamine backbone with a methyl group at the α-position relative to the nitrogen (Figure 1). Countless variation in functional group substitutions has yielded a collection of synthetic drugs with diverse pharmacological properties as stimulants, empathogens and hallucinogens [3]. ... Beyond (1R,2S)-ephedrine and (1S,2S)-pseudoephedrine, myriad other substituted amphetamines have important pharmaceutical applications. The stereochemistry at the α-carbon is often a key determinant of pharmacological activity, with (S)-enantiomers being more potent. For example, (S)-amphetamine, commonly known as d-amphetamine or dextroamphetamine, displays five times greater psychostimulant activity compared with its (R)-isomer [78]. Most such molecules are produced exclusively through chemical syntheses and many are prescribed widely in modern medicine. For example, (S)-amphetamine (Figure 4b), a key ingredient in Adderall and Dexedrine, is used to treat attention deficit hyperactivity disorder (ADHD) [79]. ...
[Figure 4](b) Examples of synthetic, pharmaceutically important substituted amphetamines. - 1 2 Glennon RA (2013). "Phenylisopropylamine stimulants: amphetamine-related agents". In Lemke TL, Williams DA, Roche VF, Zito W (eds.). Foye's principles of medicinal chemistry (7th ed.). Philadelphia, USA: Wolters Kluwer Health/Lippincott Williams & Wilkins. pp. 646–648. ISBN 9781609133450.
The simplest unsubstituted phenylisopropylamine, 1-phenyl-2-aminopropane, or amphetamine, serves as a common structural template for hallucinogens and psychostimulants. Amphetamine produces central stimulant, anorectic, and sympathomimetic actions, and it is the prototype member of this class (39).
- ↑ Lillsunde P, Korte T (March 1991). "Determination of ring- and N-substituted amphetamines as heptafluorobutyryl derivatives". Forensic Sci. Int. 49 (2): 205–213. doi:10.1016/0379-0738(91)90081-s. PMID 1855720.
- 1 2 3 Miller GM (January 2011). "The emerging role of trace amine-associated receptor 1 in the functional regulation of monoamine transporters and dopaminergic activity". J. Neurochem. 116 (2): 164–176. doi:10.1111/j.1471-4159.2010.07109.x. PMC 3005101. PMID 21073468.
- ↑ Eiden LE, Weihe E (January 2011). "VMAT2: a dynamic regulator of brain monoaminergic neuronal function interacting with drugs of abuse". Ann. N. Y. Acad. Sci. 1216 (1): 86–98. Bibcode:2011NYASA1216...86E. doi:10.1111/j.1749-6632.2010.05906.x. PMC 4183197. PMID 21272013.
- ↑ Orr K, Taylor D (2007). "Psychostimulants in the Treatment of Depression". CNS Drugs. 21 (3): 239–57. doi:10.2165/00023210-200721030-00004. PMID 17338594. S2CID 35761979.
- ↑ Dale, Elena; Bang-Andersen, Benny; Sánchez, Connie (2015). "Emerging mechanisms and treatments for depression beyond SSRIs and SNRIs". Biochemical Pharmacology. 95 (2): 81–97. doi:10.1016/j.bcp.2015.03.011. ISSN 0006-2952. PMID 25813654.
- ↑ Efforts of the National Institute on Drug Abuse to Prevent and Treat Prescription Drug Abuse Archived 29 September 2007 at the Wayback Machine, Testimony Before the Subcommittee on Criminal Justice, Drug Policy, and Human Resources Committee on Government Reform, United States House of Representatives, 26 July 2006
- ↑ Docherty, J R (7 January 2017). "Pharmacology of stimulants prohibited by the World Anti-Doping Agency (WADA)". British Journal of Pharmacology. 154 (3): 606–622. doi:10.1038/bjp.2008.124. ISSN 0007-1188. PMC 2439527. PMID 18500382.
- ↑ Caffeine for the Sustainment of Mental Task Performance: Formulations for Military Operations. Washington (DC): National Academies Press (US). 2001. Archived from the original on 9 October 2017.
- 1 2 3 "Adderall XR Prescribing Information" (PDF). United States Food and Drug Administration. June 2013. p. 11. Archived (PDF) from the original on 6 October 2014. Retrieved 7 January 2014.
- 1 2 Liddle DG, Connor DJ (June 2013). "Nutritional supplements and ergogenic AIDS". Prim. Care. 40 (2): 487–505. doi:10.1016/j.pop.2013.02.009. PMID 23668655.
Amphetamines and caffeine are stimulants that increase alertness, improve focus, decrease reaction time, and delay fatigue, allowing for an increased intensity and duration of training...
Physiologic and performance effects
• Amphetamines increase dopamine/norepinephrine release and inhibit their reuptake, leading to central nervous system (CNS) stimulation
• Amphetamines seem to enhance athletic performance in anaerobic conditions 39 40
• Improved reaction time
• Increased muscle strength and delayed muscle fatigue
• Increased acceleration
• Increased alertness and attention to task - 1 2 3 Malenka RC, Nestler EJ, Hyman SE (2009). "Chapter 13: Higher Cognitive Function and Behavioral Control". In Sydor A, Brown RY (eds.). Molecular Neuropharmacology: A Foundation for Clinical Neuroscience (2nd ed.). New York: McGraw-Hill Medical. p. 318. ISBN 978-0-07-148127-4.
Therapeutic (relatively low) doses of psychostimulants, such as methylphenidate and amphetamine, improve performance on working memory tasks both in individuals with ADHD and in normal subjects...it is now believed that dopamine and norepinephrine, but not serotonin, produce the beneficial effects of stimulants on working memory. At abused (relatively high) doses, stimulants can interfere with working memory and cognitive control, as will be discussed below. It is important to recognize, however, that stimulants act not only on working memory function, but also on general levels of arousal and, within the nucleus accumbens, improve the saliency of tasks. Thus, stimulants improve performance on effortful but tedious tasks...through indirect stimulation of dopamine and norepinephrine receptors.
- 1 2 Montgomery KA (June 2008). "Sexual desire disorders". Psychiatry. 5 (6): 50–55. PMC 2695750. PMID 19727285.
- ↑ Wilens TE, Adler LA, Adams J, Sgambati S, Rotrosen J, Sawtelle R, Utzinger L, Fusillo S (January 2008). "Misuse and diversion of stimulants prescribed for ADHD: a systematic review of the literature". J. Am. Acad. Child Adolesc. Psychiatry. 47 (1): 21–31. doi:10.1097/chi.0b013e31815a56f1. PMID 18174822.
Stimulant misuse appears to occur both for performance enhancement and their euphorogenic effects, the latter being related to the intrinsic properties of the stimulants (e.g., IR versus ER profile)...
Although useful in the treatment of ADHD, stimulants are controlled II substances with a history of preclinical and human studies showing potential abuse liability. - ↑ "Convention on psychotropic substances". United Nations Treaty Collection. United Nations. Archived from the original on 31 March 2016. Retrieved 7 January 2014.
- ↑ "Methamphetamine facts". DrugPolicy.org. Archived from the original on 17 April 2018. Retrieved 7 January 2014.
- 1 2 Chawla S, Le Pichon T (2006). "World Drug Report 2006" (PDF). United Nations Office on Drugs and Crime. pp. 128–135. Archived (PDF) from the original on 30 May 2013. Retrieved 7 January 2014.
- 1 2 Heal DJ, Smith SL, Gosden J, Nutt DJ (June 2013). "Amphetamine, past and present – a pharmacological and clinical perspective". J. Psychopharmacol. 27 (6): 479–496. doi:10.1177/0269881113482532. PMC 3666194. PMID 23539642.
- ↑ Rasmussen N (July 2006). "Making the first anti-depressant: amphetamine in American medicine, 1929–1950". J. Hist. Med. Allied Sci. 61 (3): 288–323. doi:10.1093/jhmas/jrj039. PMID 16492800. S2CID 24974454.
- ↑ "Adderall IR Prescribing Information" (PDF). United States Food and Drug Administration. March 2007. p. 5. Archived (PDF) from the original on 26 September 2013. Retrieved 2 November 2013.
- ↑ "Adderall XR Prescribing Information" (PDF). United States Food and Drug Administration. June 2013. pp. 4–8. Archived (PDF) from the original on 6 October 2014. Retrieved 7 October 2013.
- 1 2 3 4 5 6 Westfall DP, Westfall TC (2010). "Miscellaneous Sympathomimetic Agonists". In Brunton LL, Chabner BA, Knollmann BC (eds.). Goodman & Gilman's Pharmacological Basis of Therapeutics (12th ed.). New York: McGraw-Hill. ISBN 978-0-07-162442-8.
- 1 2 Shoptaw SJ, Kao U, Ling W (2009). "Treatment for amphetamine psychosis (Review)". Cochrane Database of Systematic Reviews (1): CD003026. doi:10.1002/14651858.CD003026.pub3. PMC 7004251. PMID 19160215.
- ↑ Greydanus D. "Stimulant Misuse: Strategies to Manage a Growing Problem" (PDF). American College Health Association (Review Article). ACHA Professional Development Program. p. 20. Archived from the original (PDF) on 3 November 2013. Retrieved 2 November 2013.
- ↑ Lovett R (24 September 2005). "Coffee: The demon drink?". New Scientist (2518). Archived from the original on 24 October 2007. Retrieved 3 August 2009. (subscription required)
- ↑ Nehlig, A.; Daval, J. L.; Debry, G. (1 August 2016). "Caffeine and the central nervous system: mechanisms of action, biochemical, metabolic and psychostimulant effects". Brain Research. Brain Research Reviews. 17 (2): 139–170. doi:10.1016/0165-0173(92)90012-b. PMID 1356551. S2CID 14277779.
- ↑ Bjorness, Theresa E; Greene, Robert W (8 January 2017). "Adenosine and Sleep". Current Neuropharmacology. 7 (3): 238–245. doi:10.2174/157015909789152182. ISSN 1570-159X. PMC 2769007. PMID 20190965.
- ↑ Solinas, Marcello; Ferré, Sergi; You, Zhi-Bing; Karcz-Kubicha, Marzena; Popoli, Patrizia; Goldberg, Steven R. (1 August 2002). "Caffeine Induces Dopamine and Glutamate Release in the Shell of the Nucleus Accumbens". Journal of Neuroscience. 22 (15): 6321–6324. doi:10.1523/JNEUROSCI.22-15-06321.2002. ISSN 0270-6474. PMC 6758129. PMID 12151508.
- ↑ Kamiya T, Saitoh O, Yoshioka K, Nakata H (June 2003). "Oligomerization of adenosine A2A and dopamine D2 receptors in living cells". Biochemical and Biophysical Research Communications. 306 (2): 544–9. doi:10.1016/S0006-291X(03)00991-4. PMID 12804599.
- ↑ Fishchman, N; Mello, N. Testing for Abuse Liability of Drugs in Humans (PDF). 5600 Fishers Lane Rockville, MD 20857: U.S. Department of Health and Human Services Public Health Service Alcohol, Drug Abuse, and Mental Health Administration National Institute on Drug Abuse. p. 179. Archived from the original (PDF) on 22 December 2016.
{{cite book}}: CS1 maint: location (link) - ↑ Temple JL (2009). "Caffeine use in children: what we know, what we have left to learn, and why we should worry". Neuroscience and Biobehavioral Reviews. 33 (6): 793–806. doi:10.1016/j.neubiorev.2009.01.001. PMC 2699625. PMID 19428492.
- ↑ Nkondjock A (May 2009). "Coffee consumption and the risk of cancer: an overview". Cancer Lett. 277 (2): 121–5. doi:10.1016/j.canlet.2008.08.022. PMID 18834663.
- 1 2 Arab L (2010). "Epidemiologic evidence on coffee and cancer". Nutrition and Cancer. 62 (3): 271–83. doi:10.1080/01635580903407122. PMID 20358464. S2CID 44949233.
- ↑ Santos C, Costa J, Santos J, Vaz-Carneiro A, Lunet N (2010). "Caffeine intake and dementia: systematic review and meta-analysis". J. Alzheimers Dis. 20 Suppl 1: S187–204. doi:10.3233/JAD-2010-091387. PMID 20182026.
- ↑ Marques S, Batalha VL, Lopes LV, Outeiro TF (2011). "Modulating Alzheimer's disease through caffeine: a putative link to epigenetics". J. Alzheimers Dis. 24 (2): 161–71. doi:10.3233/JAD-2011-110032. PMID 21427489.
- ↑ Arendash GW, Cao C (2010). "Caffeine and coffee as therapeutics against Alzheimer's disease". J. Alzheimers Dis. 20 Suppl 1: S117–26. doi:10.3233/JAD-2010-091249. PMID 20182037.
- ↑ Ding M, Bhupathiraju SN, Satija A, van Dam RM, Hu FB (11 February 2014). "Long-term coffee consumption and risk of cardiovascular disease: a systematic review and a dose-response meta-analysis of prospective cohort studies". Circulation. 129 (6): 643–59. doi:10.1161/circulationaha.113.005925. PMC 3945962. PMID 24201300.
- ↑ van Dam RM (2008). "Coffee consumption and risk of type 2 diabetes, cardiovascular diseases, and cancer". Applied Physiology, Nutrition, and Metabolism. 33 (6): 1269–1283. doi:10.1139/H08-120. PMID 19088789.
- ↑ Zhang Z, Hu G, Caballero B, Appel L, Chen L (June 2011). "Habitual coffee consumption and risk of hypertension: a systematic review and meta-analysis of prospective observational studies". Am. J. Clin. Nutr. 93 (6): 1212–9. doi:10.3945/ajcn.110.004044. PMID 21450934.
- ↑ Li M, Wang M, Guo W, Wang J, Sun X (March 2011). "The effect of caffeine on intraocular pressure: a systematic review and meta-analysis". Graefes Arch. Clin. Exp. Ophthalmol. 249 (3): 435–42. doi:10.1007/s00417-010-1455-1. PMID 20706731. S2CID 668498.
- ↑ Muriel P, Arauz J (2010). "Coffee and liver diseases". Fitoterapia. 81 (5): 297–305. doi:10.1016/j.fitote.2009.10.003. PMID 19825397.
- ↑ O'Connor A (2007). Never shower in a thunderstorm : surprising facts and misleading myths about our health and the world we live in (1st ed.). New York: Times Books. p. 144. ISBN 978-0-8050-8312-5. Retrieved 15 January 2014.
- ↑ Gilmore B, Michael M (February 2011). "Treatment of acute migraine headache". Am Fam Physician. 83 (3): 271–80. PMID 21302868.
- ↑ Hackett PH (2010). "Caffeine at high altitude: java at base Camp". High Alt. Med. Biol. 11 (1): 13–7. doi:10.1089/ham.2009.1077. PMID 20367483.
- ↑ Merck Manuals EPHEDrine Archived 24 March 2011 at the Wayback Machine Last full review/revision January 2010
- ↑ Meyer, Jerrold S (21 November 2013). "3,4-methylenedioxymethamphetamine (MDMA): current perspectives". Substance Abuse and Rehabilitation. 4: 83–99. doi:10.2147/SAR.S37258. ISSN 1179-8467. PMC 3931692. PMID 24648791.
- ↑ Nutt, David; King, Leslie A.; Saulsbury, William; Blakemore, Colin (24 March 2007). "Development of a rational scale to assess the harm of drugs of potential misuse". Lancet. 369 (9566): 1047–1053. doi:10.1016/S0140-6736(07)60464-4. ISSN 1474-547X. PMID 17382831. S2CID 5903121.
- ↑ Editor, Christopher Hope (7 February 2009). "Ecstasy 'no more dangerous than horse riding'". Telegraph.co.uk. Archived from the original on 10 December 2015. Retrieved 4 December 2015.
{{cite news}}:|last1=has generic name (help) - ↑ Simmler, L. D.; Buser, T. A.; Donzelli, M.; Schramm, Y; Dieu, L-H.; Huwyler, J.; Chaboz, S.; Hoener, M. C.; Liechti, M. E. (2012). "Pharmacological characterization of designer cathinones in vitro". British Journal of Pharmacology. 168 (2): 458–470. doi:10.1111/j.1476-5381.2012.02145.x. ISSN 0007-1188. PMC 3572571. PMID 22897747.
- ↑ US Patent 3478050 – 1-(3,4-methylenedioxy-phenyl)-2-pyrrolidino-alkanones
- ↑ "Abuse Of Fake 'Bath Salts' Sends Dozens To ER". KMBC.com. 23 December 2010. Archived from the original on 13 July 2011.
{{cite journal}}: Cite journal requires|journal=(help) - ↑ "MDPV Bath Salts Drug Over The Counter". Archived from the original on 10 March 2011.
- ↑ Samantha Morgan (9 November 2010). "Parents cautioned against over the counter synthetic speed". NBC 33 News. Archived from the original on 28 September 2011. Retrieved 16 May 2011.
- ↑ Kelsey Scram (6 January 2011). "Bath Salts Used to Get High". NBC 33 News. Archived from the original on 28 September 2011. Retrieved 16 May 2011.
- ↑ Cumming, E. (22 April 2010). "Mephedrone: Chemistry lessons". The Daily Telegraph. London. Archived from the original on 7 January 2014. Retrieved 14 September 2010.
- ↑ "Drugs crackdown hailed a success". BBC News. 8 March 2010. Archived from the original on 26 August 2012. Retrieved 31 March 2010.
- 1 2 3 Malenka RC, Nestler EJ, Hyman SE (2009). "15". In Sydor A, Brown RY (eds.). Molecular Neuropharmacology: A Foundation for Clinical Neuroscience (2nd ed.). New York: McGraw-Hill Medical. p. 370. ISBN 9780071481274.
Unlike cocaine and amphetamine, methamphetamine is directly toxic to midbrain dopamine neurons.
- 1 2 3 4 5 6 7 "Desoxyn Prescribing Information" (PDF). United States Food and Drug Administration. December 2013. Archived (PDF) from the original on 2 January 2014. Retrieved 6 January 2014.
- 1 2 3 4 Krasnova IN, Cadet JL (May 2009). "Methamphetamine toxicity and messengers of death". Brain Res. Rev. 60 (2): 379–407. doi:10.1016/j.brainresrev.2009.03.002. PMC 2731235. PMID 19328213.
Neuroimaging studies have revealed that METH can indeed cause neurodegenerative changes in the brains of human addicts (Aron and Paulus, 2007; Chang et al., 2007). These abnormalities include persistent decreases in the levels of dopamine transporters (DAT) in the orbitofrontal cortex, dorsolateral prefrontal cortex, and the caudate-putamen (McCann et al., 1998, 2008; Sekine et al., 2003; Volkow et al., 2001a, 2001c). The density of serotonin transporters (5-HTT) is also decreased in the midbrain, caudate, putamen, hypothalamus, thalamus, the orbitofrontal, temporal, and cingulate cortices of METH-dependent individuals (Sekine et al., 2006) ...
Neuropsychological studies have detected deficits in attention, working memory, and decision-making in chronic METH addicts ...
There is compelling evidence that the negative neuropsychiatric consequences of METH abuse are due, at least in part, to drug-induced neuropathological changes in the brains of these METH-exposed individuals ...
Structural magnetic resonance imaging (MRI) studies in METH addicts have revealed substantial morphological changes in their brains. These include loss of gray matter in the cingulate, limbic, and paralimbic cortices, significant shrinkage of hippocampi, and hypertrophy of white matter (Thompson et al., 2004). In addition, the brains of METH abusers show evidence of hyperintensities in white matter (Bae et al., 2006; Ernst et al., 2000), decreases in the neuronal marker, N-acetylaspartate (Ernst et al., 2000; Sung et al., 2007), reductions in a marker of metabolic integrity, creatine (Sekine et al., 2002) and increases in a marker of glial activation, myoinositol (Chang et al., 2002; Ernst et al., 2000; Sung et al., 2007; Yen et al., 1994). Elevated choline levels, which are indicative of increased cellular membrane synthesis and turnover are also evident in the frontal gray matter of METH abusers (Ernst et al., 2000; Salo et al., 2007; Taylor et al., 2007). - 1 2 Kuczenski R, Segal DS, Cho AK, Melega W (February 1995). "Hippocampus norepinephrine, caudate dopamine and serotonin, and behavioral responses to the stereoisomers of amphetamine and methamphetamine". J. Neurosci. 15 (2): 1308–1317. doi:10.1523/JNEUROSCI.15-02-01308.1995. PMC 6577819. PMID 7869099.
- 1 2 3 Mendelson J, Uemura N, Harris D, Nath RP, Fernandez E, Jacob P, Everhart ET, Jones RT (October 2006). "Human pharmacology of the methamphetamine stereoisomers". Clin. Pharmacol. Ther. 80 (4): 403–420. doi:10.1016/j.clpt.2006.06.013. PMID 17015058. S2CID 19072636.
- 1 2 "San Francisco Meth Zombies". Drugs, Inc. Season 4. Episode 1. 11 August 2013. 43 minutes in. ASIN B00EHAOBAO. National Geographic Channel. Archived from the original on 8 July 2016.
- ↑ United Nations Office on Drugs and Crime (2007). Preventing Amphetamine-type Stimulant Use Among Young People: A Policy and Programming Guide (PDF). New York: United Nations. ISBN 9789211482232. Archived (PDF) from the original on 16 October 2013. Retrieved 11 November 2013.
- ↑ "List of psychotropic substances under international control" (PDF). International Narcotics Control Board. United Nations. August 2003. Archived from the original (PDF) on 5 December 2005. Retrieved 19 November 2005.
- ↑ "CFR TITLE 21: DRUGS FOR HUMAN USE: PART 341 – COLD, COUGH, ALLERGY, BRONCHODILATOR, AND ANTIASTHMATIC DRUG PRODUCTS FOR OVER-THE-COUNTER HUMAN USE". United States Food and Drug Administration. April 2015. Archived from the original on 18 September 2015. Retrieved 7 March 2016.
Topical nasal decongestants --(i) For products containing levmetamfetamine identified in 341.20(b)(1) when used in an inhalant dosage form. The product delivers in each 800 milliliters of air 0.04 to 0.150 milligrams of levmetamfetamine.
- ↑ "Identification". Levomethamphetamine. Pubchem Compound. National Center for Biotechnology Information.
- ↑ Cruickshank CC, Dyer KR (July 2009). "A review of the clinical pharmacology of methamphetamine". Addiction. 104 (7): 1085–1099. doi:10.1111/j.1360-0443.2009.02564.x. PMID 19426289.
- ↑ Hart H, Radua J, Nakao T, Mataix-Cols D, Rubia K (February 2013). "Meta-analysis of functional magnetic resonance imaging studies of inhibition and attention in attention-deficit/hyperactivity disorder: exploring task-specific, stimulant medication, and age effects". JAMA Psychiatry. 70 (2): 185–198. doi:10.1001/jamapsychiatry.2013.277. PMID 23247506.
- ↑ Spencer TJ, Brown A, Seidman LJ, Valera EM, Makris N, Lomedico A, Faraone SV, Biederman J (September 2013). "Effect of psychostimulants on brain structure and function in ADHD: a qualitative literature review of magnetic resonance imaging-based neuroimaging studies". J. Clin. Psychiatry. 74 (9): 902–917. doi:10.4088/JCP.12r08287. PMC 3801446. PMID 24107764.
- ↑ Ellison, Gaylord D.; Eison, Michael S. (1983). "Continuous amphetamine intoxication: An animal model of the acute psychotic episode". Psychological Medicine. 13 (4): 751–761. doi:10.1017/S003329170005145X. PMID 6320247.
- ↑ "Efectos psicológicos del consumo de la cocaína". Avance Psicólogos (in Spanish). 2020.
- ↑ AJ Giannini; WC Price (1986). "Contemporary drugs of abuse". American Family Physician. 33: 207–213.
- ↑ Talhouth, Reinskje; Opperhuizen, Antoon; van Amsterdam G. C., Jan (October 2007). "Role of acetaldehyde in tobacco smoke addiction". European Neuropsychopharmacology. 17 (10): 627–636. doi:10.1016/j.euroneuro.2007.02.013. PMID 17382522. S2CID 25866206.
- ↑ Nutt, David J.; King, Leslie A.; Phillips, Lawrence D. (6 November 2010). "Drug harms in the UK: a multicriteria decision analysis". Lancet. 376 (9752): 1558–1565. CiteSeerX 10.1.1.690.1283. doi:10.1016/S0140-6736(10)61462-6. ISSN 1474-547X. PMID 21036393. S2CID 5667719.
- ↑ Flavahan NA (April 2005). "Phenylpropanolamine constricts mouse and human blood vessels by preferentially activating alpha2-adrenoceptors". Journal of Pharmacology and Experimental Therapeutics. 313 (1): 432–9. doi:10.1124/jpet.104.076653. PMID 15608085. S2CID 41470513.
- ↑ "Advisories, Warnings and Recalls – 2001". Health Canada. 7 January 2009. Archived from the original on 3 May 2010. Retrieved 10 January 2011.
- ↑ "Drugs Banned in India". Directorate General of Health Services, Ministry of Health and Family Welfare, Government of India. Central Drugs Standard Control Organization. Archived from the original on 13 October 2013. Retrieved 7 January 2014.
- ↑ "Benzedrex Inhaler Nasal Decongestant Inhaler". B.F. Ascher & Co., Inc. Archived from the original on 27 March 2013. Retrieved 19 December 2013.
- ↑ "Proposed Rules". Federal Register. 50 (10): 2226–2227.
- ↑ Prince v. Ascher, 90 P.3d 1020 (2004).
- ↑ Fornazzari L, Carlen PL, Kapur BM (November 1986). "Intravenous abuse of propylhexedrine (Benzedrex) and the risk of brainstem dysfunction in young adults". The Canadian Journal of Neurological Sciences. 13 (4): 337–9. doi:10.1017/S0317167100036696. PMID 2877725.
- ↑ Hunter Gillies; Wayne E. Derman; Timothy D. Noakes; Peter Smith; Alicia Evans & Gary Gabriels (1 December 1996). "Pseudoephedrine is without ergogenic effects during prolonged exercise". Journal of Applied Physiology. 81 (6): 2611–2617. doi:10.1152/jappl.1996.81.6.2611. PMID 9018513.
- ↑ Hodges, K; Hancock S; Currel K; Hamilton B; Jeukendrup AE (February 2006). "Pseudoephedrine enhances performance in 1500-m runners". Medicine and Science in Sports and Exercise. 38 (2): 329–33. doi:10.1249/01.mss.0000183201.79330.9c. PMID 16531903.
- ↑ Dickens, Charles (1856) [Digitized 19 February 2010]. "The Orsons of East Africa". Household Words: A Weekly Journal, Volume 14. Bradbury & Evans. p. 176. Retrieved 7 January 2014.
 (Free eBook)
(Free eBook) - 1 2 Al-Mugahed, Leen (October 2008). "Khat chewing in Yemen: turning over a new leaf – Khat chewing is on the rise in Yemen, raising concerns about the health and social consequences". World Health Organization. Archived from the original on 8 January 2014. Retrieved 8 January 2014.
- 1 2 Nutt D, King LA, Blakemore C (March 2007). "Development of a rational scale to assess the harm of drugs of potential misuse". Lancet. 369 (9566): 1047–53. doi:10.1016/S0140-6736(07)60464-4. PMID 17382831. S2CID 5903121.
- ↑ Haight-Ashbury Free Medical Clinic, Journal of psychoactive drugs, Volume 41, (Haight-Ashbury Publications: 2009), p.3.
- ↑ Dackis CA, Gold MS (1990). "Addictiveness of central stimulants". Advances in Alcohol & Substance Abuse. 9 (1–2): 9–26. doi:10.1300/J251v09n01_02. PMID 1974121.
- ↑ Huskinson, Sally L.; Naylor, Jennifer E.; Rowlett, James K.; Freeman, Kevin B. (7 January 2017). "Predicting abuse potential of stimulants and other dopaminergic drugs: Overview and recommendations". Neuropharmacology. 87: 66–80. doi:10.1016/j.neuropharm.2014.03.009. ISSN 0028-3908. PMC 4171344. PMID 24662599.
- ↑ Stoops, William W. (7 January 2017). "Reinforcing Effects of Stimulants in Humans: Sensitivity of Progressive-Ratio Schedules". Experimental and Clinical Psychopharmacology. 16 (6): 503–512. doi:10.1037/a0013657. ISSN 1064-1297. PMC 2753469. PMID 19086771.
- ↑ Minozzi, Silvia; Saulle, Rosella; De Crescenzo, Franco; Amato, Laura (29 September 2016). "Psychosocial interventions for psychostimulant misuse". The Cochrane Database of Systematic Reviews. 9: CD011866. doi:10.1002/14651858.CD011866.pub2. ISSN 1469-493X. PMC 6457581. PMID 27684277.
- ↑ AJ Giannini. Drug Abuse. Los Angeles, Health Information Press, 1999, pp.203–208
External links
| Look up stimulant or upper in Wiktionary, the free dictionary. |
 Media related to Stimulants at Wikimedia Commons
Media related to Stimulants at Wikimedia Commons- "Long Island Council on Alcohol & Drug Dependence – About Drugs – Stimulants". Archived from the original on 5 June 2008. Retrieved 4 August 2007.
{{cite web}}: CS1 maint: unfit URL (link) - "Online – Publications – Drugs of Abuse – Stimulants". Archived from the original on 22 September 2006. Retrieved 11 January 2008.
{{cite web}}: CS1 maint: unfit URL (link) - Asia & Pacific Amphetamine-Type Stimulants Information Centre (APAIC)