Imiloxan
Imiloxan is a drug which is used in scientific research. It acts as a selective antagonist for the α2B adrenergic receptor,[1] and has been useful for distinguishing the actions of the different α2 adrenergic subtypes.[2][3]
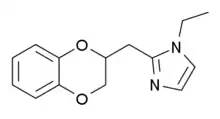 | |
| Clinical data | |
|---|---|
| ATC code |
|
| Identifiers | |
IUPAC name
| |
| CAS Number | |
| PubChem CID | |
| IUPHAR/BPS | |
| ChemSpider | |
| UNII | |
| ChEMBL | |
| CompTox Dashboard (EPA) | |
| Chemical and physical data | |
| Formula | C14H16N2O2 |
| Molar mass | 244.294 g·mol−1 |
| 3D model (JSmol) | |
SMILES
| |
InChI
| |
| | |
Synthesis
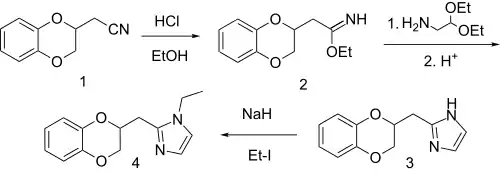
Imiloxan Synthesis:[4]
The imidazole portion of imiloxan is prepared by the reaction of an imidate with the diethyl acetal of aminoacetaldehyde. N-Alkylation of the imidazole with ethyl iodide gives imiloxan.
References
- Michel AD, Loury DN, Whiting RL (March 1990). "Assessment of imiloxan as a selective alpha 2B-adrenoceptor antagonist". British Journal of Pharmacology. 99 (3): 560–4. doi:10.1111/j.1476-5381.1990.tb12968.x. PMC 1917331. PMID 1970500.
- Cobos-Puc LE, Villalón CM, Sánchez-López A, Lozano-Cuenca J, Pertz HH, Görnemann T, Centurión D (January 2007). "Pharmacological evidence that alpha2A- and alpha2C-adrenoceptors mediate the inhibition of cardioaccelerator sympathetic outflow in pithed rats". European Journal of Pharmacology. 554 (2–3): 205–11. doi:10.1016/j.ejphar.2006.09.068. PMID 17109851.
- Romero TR, de Castro Perez A, de Francischi JN, Gama Duarte ID (April 2009). "Probable involvement of alpha(2C)-adrenoceptor subtype and endogenous opioid peptides in the peripheral antinociceptive effect induced by xylazine". European Journal of Pharmacology. 608 (1–3): 23–7. doi:10.1016/j.ejphar.2009.02.019. PMID 19236861.
- Caroon JM, Clark RD, Kluge AF, Olah R, Repke DB, Unger SH, et al. (June 1982). "Structure-activity relationships for 2-substituted imidazoles as alpha 2-adrenoceptor antagonists". Journal of Medicinal Chemistry. 25 (6): 666–70. doi:10.1021/jm00348a012. PMID 6124635.
External links
 Media related to Imiloxan at Wikimedia Commons
Media related to Imiloxan at Wikimedia Commons
This article is issued from Wikipedia. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.