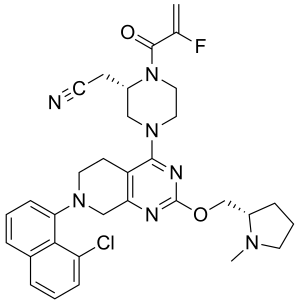
Adagrasib
 | |
| Names | |
|---|---|
| Trade names | Krazati |
| Other names | MRTX-849 |
IUPAC name
| |
| Clinical data | |
| Drug class | Antineoplastic agents |
| Main uses | Non-small cell lung cancer (NSCLC)[1] |
| Side effects | Diarrhea, nausea, musculoskeletal pain, liver problems, kidney problems, shortness of breath, low potassium, low sodium, low white blood cells, swelling[1] |
| WHO AWaRe | UnlinkedWikibase error: ⧼unlinkedwikibase-error-statements-entity-not-set⧽ |
| Routes of use | By mouth |
| Typical dose | 600 mg BID[1] |
| External links | |
| AHFS/Drugs.com | Monograph |
| Legal | |
| License data | |
| Legal status | |
| Chemical and physical data | |
| Formula | C32H35ClFN7O2 |
| Molar mass | 604.13 g·mol−1 |
| 3D model (JSmol) | |
SMILES
| |
InChI
| |
Adagrasib, sold under the brand name Krazati, is a medication used to treat non-small cell lung cancer (NSCLC).[1] Specifically it is used for KRAS G12C-mutated advanced cancer that has failed at list one other treatment.[1] It is taken by mouth.[1]
Common side effects include diarrhea, nausea, musculoskeletal pain, liver problems, kidney problems, shortness of breath, low potassium, low sodium, low white blood cells, and swelling.[1] Other side effects may include QT prolongation and pneumonitis.[1] It is an inhibitor of the RAS GTPase family.[1]
Adagrasib was approved for medical use in the United States in 2022.[1] In the United States it costs about 237,000 USD per year as of 2022.[4]
Medical uses
Adagrasib is indicated for the treatment of adults with KRAS G12C-mutated locally advanced or metastatic non-small cell lung cancer (NSCLC), as determined by an FDA approved test, who have received at least one prior systemic therapy.[2][3][5]
Dosage
It is taken at a dose of 600 mg twice per day.[1]
History
Approval by the US Food and Drug Administration (FDA) was based on KRYSTAL-1, a multicenter, single-arm, open-label clinical trial (NCT03785249) which included participants with locally advanced or metastatic non-small cell lung cancer with KRAS G12C mutations.[3] Efficacy was evaluated in 112 participants whose disease has progressed on or after platinum-based chemotherapy and an immune checkpoint inhibitor, given either concurrently or sequentially.[3]
The FDA granted the application for adagrasib fast-track, breakthrough therapy, and orphan drug designations.[3]
Research
It is undergoing clinical trials.[6][7][8][9][10][11] It is being developed by Mirati Therapeutics.[2]
References
- 1 2 3 4 5 6 7 8 9 10 11 "DailyMed - KRAZATI- adagrasib tablet, coated". dailymed.nlm.nih.gov. Archived from the original on 14 January 2023. Retrieved 12 January 2023.
- 1 2 3 "Archive copy" (PDF). Archived (PDF) from the original on 2022-12-13. Retrieved 2022-12-18.
{{cite web}}: CS1 maint: archived copy as title (link) - 1 2 3 4 5 "FDA grants accelerated approval to adagrasib for KRAS G12C-mutated NSC". U.S. Food and Drug Administration (FDA). 12 December 2022. Archived from the original on 14 December 2022. Retrieved 14 December 2022.
 This article incorporates text from this source, which is in the public domain.
This article incorporates text from this source, which is in the public domain. - ↑ "FDA Approves Rescue Combination Medication for Asthma". Formulary Watch. Archived from the original on 14 January 2023. Retrieved 12 January 2023.
- ↑ https://www.accessdata.fda.gov/drugsatfda_docs/appletter/2022/216340Orig1s000ltr.pdf Archived 2022-12-13 at the Wayback Machine
 This article incorporates text from this source, which is in the public domain.
This article incorporates text from this source, which is in the public domain. - ↑ Hallin J, Engstrom LD, Hargis L, Calinisan A, Aranda R, Briere DM, et al. (January 2020). "The KRASG12C Inhibitor MRTX849 Provides Insight toward Therapeutic Susceptibility of KRAS-Mutant Cancers in Mouse Models and Patients". Cancer Discovery. 10 (1): 54–71. doi:10.1158/2159-8290.CD-19-1167. PMC 6954325. PMID 31658955.
- ↑ Fell JB, Fischer JP, Baer BR, Blake JF, Bouhana K, Briere DM, et al. (July 2020). "Identification of the Clinical Development Candidate MRTX849, a Covalent KRASG12C Inhibitor for the Treatment of Cancer". Journal of Medicinal Chemistry. 63 (13): 6679–6693. doi:10.1021/acs.jmedchem.9b02052. PMID 32250617.
- ↑ Thein KZ, Biter AB, Hong DS (January 2021). "Therapeutics Targeting Mutant KRAS". Annual Review of Medicine. 72: 349–364. doi:10.1146/annurev-med-080819-033145. PMID 33138715. S2CID 226242453.
- ↑ Christensen JG, Olson P, Briere T, Wiel C, Bergo MO (August 2020). "Targeting Krasg12c -mutant cancer with a mutation-specific inhibitor". Journal of Internal Medicine. 288 (2): 183–191. doi:10.1111/joim.13057. PMID 32176377.
- ↑ Dunnett-Kane V, Nicola P, Blackhall F, Lindsay C (January 2021). "Mechanisms of Resistance to KRASG12C Inhibitors". Cancers. 13 (1): 151. doi:10.3390/cancers13010151. PMC 7795113. PMID 33466360.
- ↑ Jänne PA, Riely GJ, Gadgeel SM, Heist RS, Ou SI, Pacheco JM, et al. (July 2022). "Adagrasib in Non–Small-Cell Lung Cancer Harboring a KRASG12C Mutation". New England Journal of Medicine. 387 (2): 120–131. doi:10.1056/NEJMoa2204619. PMID 35658005. S2CID 249352736. Archived from the original on 2022-12-21. Retrieved 2022-12-18.
External links
| Identifiers: |
|---|
- "Adagrasib". Drug Information Portal. U.S. National Library of Medicine. Archived from the original on 2022-12-21. Retrieved 2022-12-18.
- Clinical trial number NCT03785249 for "Phase 1/2 Study of MRTX849 in Patients With Cancer Having a KRAS G12C Mutation KRYSTAL-1" at ClinicalTrials.gov