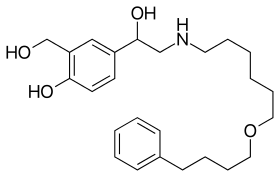
Salmeterol
 | |
| Names | |
|---|---|
| Trade names | Serevent, Aeromax, others |
| Other names | Salmeterol xinafoate |
IUPAC name
| |
| Clinical data | |
| Drug class | Long-acting β2 adrenergic receptor agonist (LABA)[1] |
| Main uses | Asthma, COPD[1] |
| Side effects | Headache, runny nose, thrush, cough, change in voice[1] |
| WHO AWaRe | UnlinkedWikibase error: ⧼unlinkedwikibase-error-statements-entity-not-set⧽ |
| Pregnancy category |
|
| Routes of use | Respiratory inhalation (Metered-dose inhaler (MDI), Dry-powder inhaler (DPI)) |
| Onset of action | About 30 min[1] |
| Duration of action | 12 hr[2] |
| External links | |
| AHFS/Drugs.com | Monograph |
| Legal | |
| License data |
|
| Legal status | |
| Pharmacokinetics | |
| Protein binding | 96% |
| Metabolism | Liver (CYP3A4) |
| Elimination half-life | 5.5 hours |
| Chemical and physical data | |
| Formula | C25H37NO4 |
| Molar mass | 415.574 g·mol−1 |
| 3D model (JSmol) | |
| Chirality | Racemic mixture |
SMILES
| |
InChI
| |
Salmeterol, sold under the brand name Serevent among others, is a medication used in the maintenance of asthma or chronic obstructive pulmonary disease (COPD).[1] In asthma it is used together with a inhaled corticosteroid.[1] It is also used for exercise-induced bronchoconstriction.[1] Effects may begin in about 30 min and last about 12 hours.[1][2]
Common side effects include headache, runny nose, thrush, cough, and change in voice.[1] Other side effects may include low potassium, anaphylaxis, bronchospasm, and QT prolongation.[1] Use during pregnancy or breastfeeding is deemed reasonable.[2] It is a long-acting β2 adrenergic receptor agonist (LABA).[1]
Salmeterol was patented in 1983 and came into medical use in 1990.[4] In the United Kingdom 120 doses costs the NHS about £30 in 2021.[2] This amount in the United States costs about 800 USD.[5] It is also avaliable as fluticasone/salmeterol.[2]
Medical uses
- Salmeterol is used in moderate-to-severe persistent asthma following previous treatment with a short-acting β2 adrenoreceptor agonist (SABA) such as salbutamol (albuterol).
- LABAs should not be used as a monotherapy, instead, they should be used concurrently with an inhaled corticosteroid, such as beclometasone dipropionate or fluticasone propionate in the treatment of asthma to minimize serious reactions such as asthma-related deaths. Combination of inhaled corticosteroids and salmeterol (LABA) has synergistic action and reduces the frequency of asthma attacks and also makes it less severe.
- In chronic obstructive pulmonary disease (COPD), LABAs may be used as monotherapy or in combination with corticosteroids. The Torch study demonstrated benefits in terms of quality of life and lung function of salmeterol alone or in combination with inhaled corticosteroids in patients with COPD[6]
- In exercise-induced bronchospasm monotherapy may be indicated in patients without persistent asthma. LABAs should not be used to treat acute symptoms.[7][8][9]
Dosage
It is used as a 50 ug dose twice per day.[1]
It is available as a dry-powder inhaler (DPI) that releases a powdered form of the drug. It was previously available as a metered-dose inhaler (MDI) but was discontinued in the US in 2002.[7][10] It is available as an MDI in other countries as of 2020.[11]
Side effects
Due to its vasodilation properties, the common side effects of salmeterol are
Other side effects
- muscle tremors,
- hypokalemia due to direct effect on beta-2 receptors on skeletal muscle.
In most cases, salmeterol side effects are minor and either do not require treatment or can easily be treated. Certain side effects, however, should be reported to a healthcare provider immediately.
Some of these more serious side effects include
- very fast heart rate,
- high blood pressure, and
- worsening breathing problems.[12]
Pregnancy and breastfeeding
Salmeterol use during pregnancy must be decided based on the risks versus benefits to the mother. There are no well-controlled studies with salmeterol in pregnant women. Some animal studies showed developmental malformation when the mother was given several clinical doses orally. In rats, salmeterol xinafoate is excreted in the milk. However, since there is no data to show excretion of salmeterol in a mother's breast milk, a decision on whether to continue or discontinue therapy should be decided based on the important benefits it provides to the mother. Pregnant and lactating women should consult their doctors before using salmeterol.[3]
Mechanism of action
Inhaled salmeterol belongs to a group of drugs called beta-2 agonists. These drugs stimulate beta-2 receptors present in the bronchial musculature. This causes them to relax and prevent the onset and worsening of symptoms of asthma. They act on the enzyme adenyl cyclase which increases the concentration of cAMP (Cyclic adenosine monophosphate). This cyclic AMP decreases the smooth muscle tone. This drug is 10,000-times more lipid soluble than the short acting beta-2 adrenoceptor agonist, albuterol. Unlike albuterol, salmeterol becomes dissolved in the lipid bilayer of the cell membrane, and its gradual dissociation from the cell membrane provides beta-2 adrenoceptors with a supply of agonist for an extended period of time.[13]
The primary noticeable difference of salmeterol from salbutamol, and other short-acting β2 adrenoreceptor agonists (SABAs), is its duration of action. Salmeterol lasts approximately 12 hours in comparison with salbutamol, which lasts about 4–6 hours.[7][8] When used regularly every day as prescribed, inhaled salmeterol decreases the number and severity of asthma attacks. However, like all LABA medications, it is not for use in relieving an asthma attack that has already started. Formoterol has been demonstrated to have a faster onset of action than salmeterol as a result of a lower lipophilicity, and has also been demonstrated to be more potent—a 12 µg dose of formoterol has been demonstrated to be equivalent to a 50 µg dose of salmeterol.[14][15]
Chemistry
Salmeterol has an aryl alkyl group with a chain length of 11 atoms from the amine. This bulkiness makes the compound more lipophilic and it also makes it selective to β2 adrenergic receptors.[16]
History


Salmeterol, first marketed and manufactured by Glaxo (now GlaxoSmithKline, GSK) in the 1980s, was released as Serevent in 1990.[10] The product is marketed by GSK under the Allen & Hanburys brand in the UK.
In November 2005, the US Food and Drug Administration (FDA) released a health advisory, alerting the public to findings that show the use of long-acting β2 agonists could lead to a worsening of symptoms, and in some cases death.[17]
While the use of inhaled LABAs are still recommended in asthma guidelines for the resulting improved symptom control,[18] further concerns have been raised. A large meta-analysis of pooled results from 19 trials with 33,826 participants, suggests that salmeterol may increase the small risks of asthma-related deaths, and this additional risk is not reduced with the additional use of inhaled steroids (e.g., as with the combination product fluticasone/salmeterol).[19] This seems to occur because although LABAs relieve asthma symptoms, they also promote bronchial inflammation and sensitivity without warning.[20]
Society and culture
Names
Combinations of inhaled steroids and these long-acting bronchodilators are becoming more widespread; the most common combination currently in use is fluticasone/salmeterol (brand names Seretide (UK) and Advair (US)). Another combination is budesonide/formoterol (brand name Symbicort).[21]
See also
- Vilanterol — an ultra-long-acting β2 adrenoreceptor agonist with a similar chemical structure.
- Formoterol
- Bambuterol
References
- 1 2 3 4 5 6 7 8 9 10 11 12 "Salmeterol". American Society of Health-System Pharmacists. Archived from the original on 25 July 2021. Retrieved 10 October 2021.
- 1 2 3 4 5 BNF (80 ed.). BMJ Group and the Pharmaceutical Press. September 2020 – March 2021. p. 266. ISBN 978-0-85711-369-6.
{{cite book}}: CS1 maint: date format (link) - 1 2 "Serevent Diskus- salmeterol xinafoate powder, metered". DailyMed. 13 January 2020. Archived from the original on 14 December 2020. Retrieved 6 September 2020.
- ↑ Fischer J, Ganellin CR (2006). Analogue-based Drug Discovery. John Wiley & Sons. p. 543. ISBN 9783527607495. Archived from the original on 2021-07-24. Retrieved 2021-06-21.
- ↑ "Salmeterol Xinafoate Prices, Coupons & Savings Tips - GoodRx". GoodRx. Retrieved 10 October 2021.
- ↑ Calverley PM, Anderson JA, Celli B, Ferguson GT, Jenkins C, Jones PW, et al. (February 2007). "Salmeterol and fluticasone propionate and survival in chronic obstructive pulmonary disease". The New England Journal of Medicine. 356 (8): 775–89. doi:10.1056/NEJMoa063070. PMID 17314337.
- 1 2 3 "global initiative for chronic obstructive disease" (PDF). goldcopd.org. Archived from the original (PDF) on 24 September 2015. Retrieved 30 October 2014.
- 1 2 "Global initiative for asthma" (PDF). ginasthma.org. Archived from the original (PDF) on 22 August 2014. Retrieved 30 October 2014.
- ↑ "Use of long-acting beta agonist in chronic obstructive pulmonary disease". mhra.gov.uk. Archived from the original on 3 November 2014. Retrieved 30 October 2014.
- 1 2 "Benefit Risk Assessment of Salmeterol for the Treatment of Asthma in Adults and Children" (PDF). fda.gov. Archived from the original (PDF) on 26 January 2018.
- ↑ "Serevent Dosieraeros 25 mcg FCKW-frei". compendium.ch (in Deutsch). Archived from the original on 2021-07-24. Retrieved 2021-06-21.
- ↑ "Medtv". HealthSavy. Archived from the original on 22 September 2013. Retrieved 8 March 2012.
- ↑ XPharm : the comprehensive pharmacology reference. Enna, S. J., Bylund, David B., Elsevier Science (Firm). Amsterdam: Elsevier. 2008. ISBN 978-0-08-055232-3. OCLC 712018683. Archived from the original on 2021-08-29. Retrieved 2021-06-21.
{{cite book}}: CS1 maint: others (link) - ↑ "National Asthma Education and Prevention Program". Archived from the original on 3 November 2014. Retrieved 30 October 2014.
- ↑ "Recommended Medication for Asthma" (PDF). www.partnershiphp.org. Archived from the original (PDF) on 2014-11-03.
- ↑ Mehta A. "Medicinal Chemistry of Adrenergics and Cholinergics". PharmaXChange. Archived from the original on 2010-11-04.
- ↑ "Advair Diskus, Advair HFA, Brovana, Foradil, Perforomist, Serevent Diskus, and Symbicort Information (Long Acting Beta Agonists)". Fierce Biotech. 6 March 2008. Archived from the original on 29 August 2021. Retrieved 21 June 2021.
- ↑ British Thoracic Society & Scottish Intercollegiate Guidelines Network (SIGN). British Guideline on the Management of Asthma. Guideline No. 63. Edinburgh:SIGN; 2004. (HTML Archived 2006-06-18 at the Wayback Machine, Full PDF Archived 2006-07-24 at the Wayback Machine, Summary PDF Archived 2006-07-24 at the Wayback Machine)
- ↑ Salpeter SR, Buckley NS, Ormiston TM, Salpeter EE (June 2006). "Meta-analysis: effect of long-acting beta-agonists on severe asthma exacerbations and asthma-related deaths". Annals of Internal Medicine. 144 (12): 904–12. doi:10.7326/0003-4819-144-12-200606200-00126. PMID 16754916.
- ↑ Ramanujan K (June 9, 2006). "Common asthma inhalers cause up to 80 percent of asthma-related deaths, Cornell and Stanford researchers assert". ChronicalOnline - Cornell University. Archived from the original on March 4, 2013. Retrieved June 21, 2021.
- ↑ "Australian Medicines Handbook". amhonline.amh.net.au. Archived from the original on 2020-05-25. Retrieved 2020-05-07.
External links
| External sites: |
|
|---|---|
| Identifiers: |