Norethisterone enanthate
 | |
 | |
| Names | |
|---|---|
| Trade names | Noristerat, others |
| Other names | NETE; NET-EN; Norethindrone enanthate; SH-393; 17α-Ethynyl-19-nortestosterone 17β-enanthate; 17α-Ethynylestra-4-en-17β-ol-3-one 17β-enanthate |
IUPAC name
| |
| Clinical data | |
| Drug class | Progestogen; Progestin; Progestogen ester |
| WHO AWaRe | UnlinkedWikibase error: ⧼unlinkedwikibase-error-statements-entity-not-set⧽ |
| Routes of use | Intramuscular injection |
| Defined daily dose | not established[1] |
| External links | |
| AHFS/Drugs.com | International Drug Names |
| Legal | |
| Legal status |
|
| Chemical and physical data | |
| Formula | C27H38O3 |
| Molar mass | 410.598 g·mol−1 |
| 3D model (JSmol) | |
SMILES
| |
InChI
| |
Norethisterone enanthate (NETE), also known as norethindrone enanthate, is a form of progestogen-only injectable birth control which is used to prevent pregnancy in women.[2][3][4] It may be used following childbirth, miscarriage, or abortion.[2] The failure rate per year in preventing pregnancy is 2 per 100 women.[5] Each dose lasts two months with only up to two doses typically recommended.[6][2]
Side effects include breast pain, headaches, depression, irregular menstrual periods, and pain at the site of injection.[6] Use in those with liver disease is not recommended as is use during pregnancy due to risk of birth defects.[2] Use appears to be okay during breastfeeding.[2] It does not protect against sexually transmitted infections.[2] NETE is an ester and prodrug of norethisterone,[7] through which it works.[2] It works as a method of birth control by stopping ovulation.[2]
Norethisterone was patented in 1951 and came into medical use in 1957.[8][9] It is on the World Health Organization's List of Essential Medicines.[10] The wholesale cost in the developing world is about US$1.04–7.99 per 200 mg vial.[11] It has been approved by itself in more than 60 countries including the United Kingdom and some in Europe, Central America, and Africa, and in combination with estradiol valerate in at least 36 countries mainly in Latin America.[5][12][13][14] It is not available in the United States.[12]
Medical uses
NETE is used on its own as a long-lasting progestogen-only injectable contraceptive in women.[2][6] It is administered via intramuscular injection once every two months.[2][6]
Dosage
The defined daily dose is not established[1]
Contraindications
Side effects
Side effects of NETE may include breast pain, headaches, depression, irregular menstrual periods, and pain at the site of injection.[6] It can cause birth defects in the fetus if used during pregnancy.[2]
Overdose
Interactions
Pharmacology
Pharmacodynamics
NETE is a prodrug of norethisterone in the body.[15] Upon reaching circulation, it is rapidly converted into norethisterone by esterases. Hence, as a prodrug of norethisterone, NETE has essentially the same effects as norethisterone, acting as a potent progestogen with additional weak androgenic and estrogenic activity (the latter via its metabolite ethinylestradiol).[16] NETA has some progestogenic activity of its own, but it is unclear if NETE does similarly.[15]
NETE is of about 38% higher molecular weight than norethisterone due to the presence of its C17β enanthate ester.[3]
| Compound | Typea | PR | AR | ER | GR | MR | SHBG | CBG |
|---|---|---|---|---|---|---|---|---|
| Norethisterone | – | 67–75 | 15 | 0 | 0–1 | 0–3 | 16 | 0 |
| 5α-Dihydronorethisterone | Metabolite | 25 | 27 | 0 | 0 | ? | ? | ? |
| 3α,5α-Tetrahydronorethisterone | Metabolite | 1 | 0 | 0–1 | 0 | ? | ? | ? |
| 3α,5β-Tetrahydronorethisterone | Metabolite | ? | 0 | 0 | ? | ? | ? | ? |
| 3β,5α-Tetrahydronorethisterone | Metabolite | 1 | 0 | 0–8 | 0 | ? | ? | ? |
| Ethinylestradiol | Metabolite | 15–25 | 1–3 | 112 | 1–3 | 0 | 0.18 | 0 |
| Norethisterone acetate | Prodrug | 20 | 5 | 1 | 0 | 0 | ? | ? |
| Norethisterone enanthate | Prodrug | ? | ? | ? | ? | ? | ? | ? |
| Noretynodrel | Prodrug | 6 | 0 | 2 | 0 | 0 | 0 | 0 |
| Etynodiol | Prodrug | 1 | 0 | 11–18 | 0 | ? | ? | ? |
| Etynodiol diacetate | Prodrug | 1 | 0 | 0 | 0 | 0 | ? | ? |
| Lynestrenol | Prodrug | 1 | 1 | 3 | 0 | 0 | ? | ? |
| Notes: Values are percentages (%). Reference ligands (100%) were promegestone for the PR, metribolone for the AR, estradiol for the ER, dexamethasone for the GR, aldosterone for the MR, dihydrotestosterone for SHBG, and cortisol for CBG. Footnotes: a = Active or inactive metabolite, prodrug, or neither of norethisterone. Sources: See template. | ||||||||
| Progestogen | Form | Major brand names | Class | TFD (14 days) | POIC-D (2–3 months) | CIC-D (month) | Duration | |
|---|---|---|---|---|---|---|---|---|
| Algestone acetophenide | Oil solution | Perlutal, Topasel, Yectames | Pregnane | ? | – | 75–150 mg | 100 mg ≈ 14–32 days | |
| Cyproterone acetate | Oil solution | Androcur Depot | Pregnane | ? | – | – | 300 mg ≈ 20 days | |
| Dydrogesteronea | Aqueous suspension | – | Retropregnane | ? | – | – | 100 mg ≈ 16–38 days | |
| Gestonorone caproate | Oil solution | Depostat, Primostat | Norpregnane | 25–50 mg | – | – | 25–50 mg ≈ 8–13 days | |
| Hydroxyprogesterone acetatea | Aqueous suspension | – | Pregnane | 350 mg | – | – | 150–350 mg ≈ 9–16 days | |
| Hydroxyprogesterone caproate | Oil solution | Delalutin, Proluton, Makena | Pregnane | 250–500 mgb | – | 250–500 mg | 65–500 mg ≈ 5–21 days | |
| Levonorgestrel butanoatea | Aqueous suspension | – | Gonane | ? | – | – | 5–50 mg ≈ 3–6 months | |
| Lynestrenol phenylpropionatea | Oil solution | – | Estrane | ? | – | – | 50–100 mg ≈ 14–30 days | |
| Medroxyprogesterone acetate | Aqueous suspension | Depo-Provera | Pregnane | 50–100 mg | 150 mg | 25 mg | 50–150 mg ≈ 14–50+ days | |
| Megestrol acetate | Aqueous suspension | Mego-E | Pregnane | ? | – | 25 mg | 25 mg ≈ >14 daysc | |
| Norethisterone enanthate | Oil solution | Noristerat, Mesigyna | Estrane | 100–200 mg | 200 mg | 50 mg | 50–200 mg ≈ 11–52 days | |
| Oxogestone phenylpropionatea | Oil solution | – | Norpregnane | ? | – | – | 100 mg ≈ 19–20 days | |
| Progesterone | Oil solution | Progestaject, Gestone, Strone | Pregnane | 200 mgb | – | – | 25–350 mg ≈ 2–6 days | |
| Aqueous suspension | Agolutin Depot | Pregnane | 50–200 mg | – | – | 50–300 mg ≈ 7–14 days | ||
| Note: All by intramuscular or subcutaneous injection. All are synthetic except for P4, which is bioidentical. P4 production during the luteal phase is ~25 (15–50) mg/day. The OID of OHPC is 250 to 500 mg/month. Footnotes: a = Never marketed by this route. b = In divided doses (2 × 125 or 250 mg for OHPC, 10 × 20 mg for P4). c = Half-life is ~14 days. Sources: Main: See template. | ||||||||
Pharmacokinetics
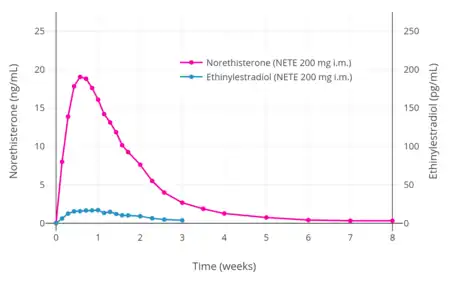
A single intramuscular injection of 50 to 200 mg NETE in oil solution has been found to have a duration of action of 11 to 52 days in terms of clinical biological effect in the uterus and on body temperature in women.[18]
Similarly to oral norethisterone and norethisterone acetate, intramuscular NETE has been found to form ethinylestradiol as an active metabolite.[17] With a single intramuscular injection of 200 mg NETE in premenopausal women, the mean maximum concentration of ethinylestradiol was 32% of that of a combined oral contraceptive containing 30 μg ethinylestradiol, the maximum equivalent oral dose of ethinylestradiol observed in the first few days of exposure was 20.3 μg/day, and the mean equivalent oral dose of ethinylestradiol over 8 weeks was 4.41 μg/day.[17] As such, the exposure to ethinylestradiol was described as markedly lower than that of an oral contraceptive containing 30 μg ethinylestradiol.[17] The estimated conversion rate of NETE into ethinylestradiol was 0.1%, which was much lower than that observed for oral norethisterone and norethisterone enanthate (0.2–1.0%), likely due to the lack of the first pass through the liver with parenteral administration.[17] In accordance with the low levels of ethinylestradiol produced, no increase rates of thromboembolism or hepatic adenoma have been observed in post-authorization data of intramuscular NETE, and the medication does not resemble combined oral contraceptives containing ethinylestradiol in its safety profile.[17]
Chemistry
NETE, also known as norethinyltestosterone enanthate, as well as 17α-ethynyl-19-nortestosterone 17β-enanthate or 17α-ethynylestr-4-en-17β-ol-3-one 17β-enanthate, is a progestin, or synthetic progestogen, of the 19-nortestosterone group, and a synthetic estrane steroid.[3][4] It is the C17β enanthate ester of norethisterone.[3][4] NETE is a derivative of testosterone with an ethynyl group at the C17α position, the methyl group at the C19 position removed, and an enanthate ester attached at the C17β position.[3][4] In addition to testosterone, it is a combined derivative of nandrolone (19-nortestosterone) and ethisterone (17α-ethynyltestosterone).[3][4] Esters related to NETE include norethisterone acetate and levonorgestrel butanoate.[3][4]
History
NETE was introduced by Schering as Noristerat in 1957.[9] It was the second long-acting progestogen to be used clinically, after hydroxyprogesterone caproate.[19] The medication was the first progestogen-only injectable contraceptive, preceding medroxyprogesterone acetate (Depo-Provera).[9]
Society and culture
Generic names
Norethisterone enantate is the generic name of the drug and its INNM and BANM.[3][4][20][21][22] It is also spelled as norethisterone enanthate and is also known as norethindrone enanthate (the USAN of norethisterone being norethindrone).[3][4][20][21][22] NETE is known by its former developmental code name SH-393 as well.[3][4][20][21][22]
Brand names
NETE has been marketed alone as a progestogen-only injectable contraceptive under the brand names Depocon, Doryxas, NET-EN, Noristat, Noristerat, Norigest, and Nur-Isterate, and in combination with estradiol valerate as a combined injectable contraceptive under the brand names Chinese Injectable No. 3, Efectimes, Ginediol, Mesigyna, Mesilar, Meslart, Mesocept, Mesygest, Nofertyl, Nofertyl Lafrancol, Noregyna, Norestrin, Norifam, Norigynon, Nostidyn, Sexseg, and Solouna.[4][21][22][23]
| Composition | Dose | Brand names | Use |
|---|---|---|---|
| NET only | Low (e.g., 0.35 mg) | Multiple[lower-alpha 1] | Progestogen-only oral contraceptive |
| NET or NETA only | High (e.g., 5 mg, 10 mg) | Multiple[lower-alpha 2] | Gynecological disorders and other uses |
| NETE only | Injection (e.g., 200 mg) | Multiple[lower-alpha 3] | Progestogen-only injectable contraceptive |
| NET or NETA with ethinylestradiol | Low (e.g., 0.4 mg, 0.5 mg, 0.75 mg, 1 mg, 1.5 mg) | Multiple[lower-alpha 4] | Combined oral contraceptive |
| NET with mestranol | Low (e.g., 1 mg, 2 mg) | Multiple[lower-alpha 5] | Combined oral contraceptive |
| NETA with estradiol | Low (e.g., 0.1 mg, 0.5 mg) | Multiple[lower-alpha 6] | Combined menopausal hormone therapy |
| NETE with estradiol valerate | Injection (e.g., 50 mg) | Multiple[lower-alpha 7] | Combined injectable contraceptive |
| Abbreviations: NET = Norethisterone. NETA = Norethisterone acetate. NETE = Norethisterone enanthate. Sources: [24][25][4][26] Notes:
| |||
Availability
NETE has been approved for use alone as a progestogen-only injectable contraceptive in more than 60 countries throughout the world including in Europe, Latin America, Asia, and Africa.[5][12][13] Specific countries in which NETE as a standalone medication is or has been available include Bangladesh, France, Germany, India, Italy, Malaysia, Mexico, the Philippines, Singapore, South Africa, Thailand, and the United Kingdom.[4][21][22][23]
NETE has been approved for use in combination with estradiol valerate as a combined injectable contraceptive in at least 36 countries, mostly in Latin America but also in Africa.[13][14] It is or has been available in combination with estradiol valerate in Argentina, the Bahamas, Barbados, Bolivia, Brazil, Chile, Colombia, Costa Rica, the Dominican Republic, Ecuador, Egypt, El Salvador, Ghana, Grenada, Guatemala, Guyana, Haiti, Honduras, Jamaica, Kenya, Mexico, Nicaragua, Panama, Paraguay, Peru, St. Lucia, Turkey, Uruguay, Venezuela, and Zimbabwe.[21][22][23][16]
NETE is not available in any form in the United States.[12]
Research
NETE was studied by Schering for use as a progestogen-only injectable contraceptive at a dose of 25 mg once a month but produced poor cycle control with this regimen and was not marketed.[27]
NETE has been studied for use as a potential male hormonal contraceptive in combination with testosterone in men.[28]
See also
References
- 1 2 "WHOCC - ATC/DDD Index". www.whocc.no. Archived from the original on 1 July 2021. Retrieved 15 September 2020.
- 1 2 3 4 5 6 7 8 9 10 11 "Noristerat 200mg, solution for intramuscular injection - Summary of Product Characteristics (SPC) - (eMC)". www.medicines.org.uk. Archived from the original on 31 December 2016. Retrieved 31 December 2016.
- 1 2 3 4 5 6 7 8 9 10 J. Elks (14 November 2014). The Dictionary of Drugs: Chemical Data: Chemical Data, Structures and Bibliographies. Springer. pp. 886–. ISBN 978-1-4757-2085-3. Archived from the original on 5 November 2017.
- 1 2 3 4 5 6 7 8 9 10 11 12 Index Nominum 2000: International Drug Directory. Taylor & Francis US. 2000. p. 750. ISBN 978-3-88763-075-1. Archived from the original on 28 May 2013. Retrieved 30 May 2012.
- 1 2 3 Committee on Contraceptive Development (U.S.) (1 January 1990). Luigi Mastroianni; Peter J. Donaldson; Thomas T. Kane (eds.). Developing New Contraceptives: Obstacles and Opportunities. National Academies. pp. 38–. NAP:14119.
- 1 2 3 4 5 World Health Organization (2009). Stuart MC, Kouimtzi M, Hill SR (eds.). WHO Model Formulary 2008. World Health Organization. p. 370. hdl:10665/44053. ISBN 9789241547659.
- ↑ Wu L, Janagam DR, Mandrell TD, Johnson JR, Lowe TL (2015). "Long-acting injectable hormonal dosage forms for contraception". Pharmaceutical Research. 32 (7): 2180–91. doi:10.1007/s11095-015-1686-2. PMID 25899076.
- ↑ Fischer, Janos; Ganellin, C. Robin (2006). Analogue-based Drug Discovery. John Wiley & Sons. p. 478. ISBN 9783527607495. Archived from the original on 2016-12-20.
- 1 2 3 Vern L. Bullough (2001). Encyclopedia of Birth Control. ABC-CLIO. pp. 145–. ISBN 978-1-57607-181-6. Archived from the original on 2017-11-05.
- ↑ World Health Organization (2019). World Health Organization model list of essential medicines: 21st list 2019. Geneva: World Health Organization. hdl:10665/325771. WHO/MVP/EMP/IAU/2019.06. License: CC BY-NC-SA 3.0 IGO.
- ↑ "Norethisterone". International Drug Price Indicator Guide. Archived from the original on 22 January 2018. Retrieved 8 December 2016.
- 1 2 3 4 Amy Whitaker; Melissa Gilliam (27 June 2014). Contraception for Adolescent and Young Adult Women. Springer. p. 96. ISBN 978-1-4614-6579-9. Archived from the original on 5 November 2017.
- 1 2 3 Bagade O, Pawar V, Patel R, Patel B, Awasarkar V, Diwate S (2014). "Increasing use of long-acting reversible contraception: safe, reliable, and cost-effective birth control" (PDF). World J Pharm Pharm Sci. 3 (10): 364–392. ISSN 2278-4357. Archived from the original (PDF) on 2017-08-10. Retrieved 2018-08-02.
- 1 2 Newton JR, D'arcangues C, Hall PE (1994). "A review of "once-a-month" combined injectable contraceptives". J Obstet Gynaecol (Lahore). 4 Suppl 1: S1–34. doi:10.3109/01443619409027641. PMID 12290848.
- 1 2 Hapgood JP, Koubovec D, Louw A, Africander D (November 2004). "Not all progestins are the same: implications for usage". Trends Pharmacol. Sci. 25 (11): 554–7. doi:10.1016/j.tips.2004.09.005. PMID 15491776.
- 1 2 IARC Working Group on the Evaluation of Carcinogenic Risks to Humans; World Health Organization; International Agency for Research on Cancer (2007). Combined Estrogen-progestogen Contraceptives and Combined Estrogen-progestogen Menopausal Therapy. World Health Organization. pp. 417, 432. ISBN 978-92-832-1291-1. Archived from the original on 2017-11-05.
Norethisterone and its acetate and enanthate esters are progestogens that have weak estrogenic and androgenic properties.
- 1 2 3 4 5 6 Friedrich C, Berse M, Klein S, Rohde B, Höchel J (March 2018). "In Vivo Formation of Ethinylestradiol After Intramuscular Administration of Norethisterone Enantate". J Clin Pharmacol. 58 (6): 781–789. doi:10.1002/jcph.1079. PMID 29522253.
- ↑ J. Ferin (September 1972). "Effects, Duration of Action and Metabolism in Man". In M. Tausk (ed.). Pharmacology of the Endocrine System and Related Drugs: Progesterone, Progestational Drugs and Antifertility Agents. Vol. II. Pergamon Press. pp. 13–24. ISBN 978-0080168128. OCLC 278011135. Archived from the original on 2021-04-12. Retrieved 2019-05-19.
- ↑ Boschann HW (July 1958). "Observations of the role of progestational agents in human gynecologic disorders and pregnancy complications". Ann. N. Y. Acad. Sci. 71 (5): 727–52. doi:10.1111/j.1749-6632.1958.tb46803.x. PMID 13583829.
- 1 2 3 I.K. Morton; Judith M. Hall (6 December 2012). Concise Dictionary of Pharmacological Agents: Properties and Synonyms. Springer Science & Business Media. pp. 201–. ISBN 978-94-011-4439-1. Archived from the original on 15 February 2017. Retrieved 16 September 2018.
- 1 2 3 4 5 6 "Archive copy". Archived from the original on 2018-09-15. Retrieved 2018-09-16.
{{cite web}}: CS1 maint: archived copy as title (link) - 1 2 3 4 5 6 Sweetman, Sean C., ed. (2009). "Sex hormones and their modulators". Martindale: The Complete Drug Reference (36th ed.). London: Pharmaceutical Press. p. 2082. ISBN 978-0-85369-840-1. Archived from the original on 2021-08-28. Retrieved 2018-09-16.
- 1 2 3 "Archive copy". Archived from the original on 2019-05-23. Retrieved 2018-09-16.
{{cite web}}: CS1 maint: archived copy as title (link) - ↑ https://www.drugs.com/international/norethisterone.html
- ↑ "Drugs@FDA: FDA Approved Drug Products". United States Food and Drug Administration. Retrieved 27 November 2016.
- ↑ IARC Working Group on the Evaluation of Carcinogenic Risks to Humans; International Agency for Research on Cancer (1 January 1999). Hormonal Contraception and Post-menopausal Hormonal Therapy (PDF). IARC. p. 65. ISBN 978-92-832-1272-0. Lay summary.
{{cite book}}: Cite uses deprecated parameter|lay-url=(help) - ↑ Toppozada M (June 1977). "The clinical use of monthly injectable contraceptive preparations". Obstet Gynecol Surv. 32 (6): 335–47. doi:10.1097/00006254-197706000-00001. PMID 865726.
- ↑ Nieschlag E (2010). "Clinical trials in male hormonal contraception" (PDF). Contraception. 82 (5): 457–70. doi:10.1016/j.contraception.2010.03.020. PMID 20933120. Archived (PDF) from the original on 2020-12-05. Retrieved 2019-07-11.
External links
| Identifiers: |
|---|
- "Norethisterone Enanthate". Drug Information Portal. U.S. National Library of Medicine. Archived from the original on 2021-08-28. Retrieved 2020-05-17.