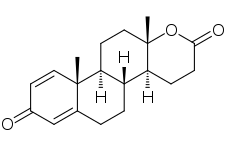
Testolactone
 | |
| Clinical data | |
|---|---|
| Trade names | Teslac |
| Other names | 13-Hydroxy-3-oxo-13,17-secoandrosta-1,4-dien-17-oic acid δ-lactone; SQ-9538; Fludestrin; NSC-12173; NSC-23759 |
| AHFS/Drugs.com | Consumer Drug Information |
| Routes of administration | By mouth |
| Drug class | Aromatase inhibitor; Antiestrogen |
| ATC code |
|
| Pharmacokinetic data | |
| Protein binding | ~85% |
| Metabolism | Liver |
| Excretion | Urine |
| Identifiers | |
IUPAC name
| |
| CAS Number | |
| PubChem CID | |
| IUPHAR/BPS | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEBI | |
| ChEMBL | |
| CompTox Dashboard (EPA) | |
| ECHA InfoCard | 100.012.304 |
| Chemical and physical data | |
| Formula | C19H24O3 |
| Molar mass | 300.398 g·mol−1 |
| 3D model (JSmol) | |
SMILES
| |
| | |
Testolactone (INN, USAN) (brand name Teslac) is a non-selective, irreversible, steroidal aromatase inhibitor which is used as an antineoplastic drug to treat advanced-stage breast cancer.[1][2][3][4] The drug was discontinued in 2008 and is no longer available for medical use.[4] However, it has been reported to still be marketed in the United States by Bristol-Myers Squibb under the brand name Teslac.[5]
Medical uses
Testolactone is mainly used for treating various types of breast cancer in women who have been through menopause or whose ovaries no longer function.[6] It works by blocking the production of estrogens, which helps prevent the growth of breast cancers that are stimulated by estrogens. It may also prevent tumor cells from being activated by other hormones.[6] Testolactone has also been used to postpone precocious puberty because of its ability to block estrogen production.[7] In addition, it has been used in the treatment of gynecomastia.[8][9]
Testolactone is used to treat breast cancer at a dosage of 250 mg four times per day by mouth or 100 mg three times per week by intramuscular injection.[10]
Available forms
Testolactone has been provided in the form of 50 mg and 250 mg oral tablets.[11][12]
Side effects
The most common side effects include:
- Abnormal skin sensations
- Aches of the legs and arms
- General body discomfort
- Hair loss
- Loss of appetite
- Nausea
- Redness of the tongue
- Vomiting
Pharmacology
The principal action of testolactone is reported to be inhibition of aromatase activity and the reduction in estrogen synthesis that follows. Androstenedione, a 19-carbon steroid hormone produced in the adrenal glands and the gonads, is where estrone synthesis originates and is the source of estrogen in postmenopausal women. In vitro studies report that the aromatase inhibition may be noncompetitive and irreversible, and could possibly account for the persistence of this drug's effect on estrogen synthesis after drug withdrawal.[2] Testolactone at a dosage of 1,000 mg/day has been found to decrease estradiol levels in men by 25 to 50% after 6 to 10 days of use.[12] This reduction is substantially less than with second- and third-generation aromatase inhibitors.[12]
In addition to its activity as an aromatase inhibitor, testolactone also reportedly possesses some anabolic activity and weak androgenic activity via binding to and activation of the androgen receptor (AR).[4] However, its affinity for the AR is very low; in one study, it showed 0.0029% of the affinity of the anabolic steroid metribolone (100%) for the human AR (Ki = 41 μM and 1.18 nM, respectively).[13] In accordance, androgenic side effects such as hirsutism, acne, and voice changes have been reported in no women in clinical trials with testolactone.[10]
| Generation | Medication | Dosage | % inhibitiona | Classb | IC50c |
|---|---|---|---|---|---|
| First | Testolactone | 250 mg 4x/day p.o. | ? | Type I | ? |
| 100 mg 3x/week i.m. | ? | ||||
| Rogletimide | 200 mg 2x/day p.o. 400 mg 2x/day p.o. 800 mg 2x/day p.o. | 50.6% 63.5% 73.8% | Type II | ? | |
| Aminoglutethimide | 250 mg mg 4x/day p.o. | 90.6% | Type II | 4,500 nM | |
| Second | Formestane | 125 mg 1x/day p.o. 125 mg 2x/day p.o. 250 mg 1x/day p.o. | 72.3% 70.0% 57.3% | Type I | 30 nM |
| 250 mg 1x/2 weeks i.m. 500 mg 1x/2 weeks i.m. 500 mg 1x/1 week i.m. | 84.8% 91.9% 92.5% | ||||
| Fadrozole | 1 mg 1x/day p.o. 2 mg 2x/day p.o. | 82.4% 92.6% | Type II | ? | |
| Third | Exemestane | 25 mg 1x/day p.o. | 97.9% | Type I | 15 nM |
| Anastrozole | 1 mg 1x/day p.o. 10 mg 1x/day p.o. | 96.7–97.3% 98.1% | Type II | 10 nM | |
| Letrozole | 0.5 mg 1x/day p.o. 2.5 mg 1x/day p.o. | 98.4% 98.9%–>99.1% | Type II | 2.5 nM | |
| Footnotes: a = In postmenopausal women. b = Type I: Steroidal, irreversible (substrate-binding site). Type II: Nonsteroidal, reversible (binding to and interference with the cytochrome P450 heme moiety). c = In breast cancer homogenates. Sources: See template. | |||||
Chemistry
Testolactone, also known as 13-hydroxy-3-oxo-13,17-secoandrosta-1,4-dien-17-oic acid δ-lactone, is a synthetic 18-oxasteroid and a D-homo-18-oxo analogue of androstenedione (androst-4-en-3,17-dione), with a six-membered lactone ring in place of the five-membered carbocyclic D-ring.[4][1]
History
Testolactone was first approved for medical use in the United States in 1970.[12]
References
- 1 2 George W.A Milne (8 May 2018). Drugs: Synonyms and Properties: Synonyms and Properties. Taylor & Francis. pp. 935–. ISBN 978-1-351-78989-9.
- 1 2 Testolactone at DrugBank.ca
- ↑ Dunkel L (July 2006). "Use of aromatase inhibitors to increase final height". Mol. Cell. Endocrinol. 254–255: 207–16. doi:10.1016/j.mce.2006.04.031. PMID 16766117. S2CID 34706246.
- 1 2 3 4 Thomas L. Lemke; David A. Williams (24 January 2012). Foye's Principles of Medicinal Chemistry. Lippincott Williams & Wilkins. pp. 1362–. ISBN 978-1-60913-345-0.
- ↑ "Testolactone Advanced Patient Information".
- 1 2 Testolactone facts and comparisons at Drugs.com
- ↑ Carel, J.-C.; Lahlou, N; Roger, M; Chaussain, JL (2004). "Precocious puberty and statural growth". Human Reproduction Update. 10 (2): 135–47. doi:10.1093/humupd/dmh012. PMID 15073143.
- ↑ Kenneth L. Becker (2001). Principles and Practice of Endocrinology and Metabolism. Lippincott Williams & Wilkins. pp. 1206–. ISBN 978-0-7817-1750-2.
- ↑ Kirby I. Bland; Edward M. Copeland; V. Suzanne Klimberg (9 September 2009). The Breast E-Book: Comprehensive Management of Benign and Malignant Diseases. Elsevier Health Sciences. pp. 162–. ISBN 978-1-4377-1121-9.
- 1 2 Aurel Lupulescu (24 October 1990). Hormones and Vitamins in Cancer Treatment. CRC Press. pp. 57, 64. ISBN 978-0-8493-5973-6.
- ↑ Medical Economics (February 1983). Physicians Desk Reference. PDR Network, LLC. pp. 1921, 1963. ISBN 978-0-87489-859-0.
- 1 2 3 4 William Llewellyn (2011). Anabolics. Molecular Nutrition Llc. pp. 805–. ISBN 978-0-9828280-1-4.
- ↑ Eil C, Edelson SK (July 1984). "The use of human skin fibroblasts to obtain potency estimates of drug binding to androgen receptors". J. Clin. Endocrinol. Metab. 59 (1): 51–5. doi:10.1210/jcem-59-1-51. PMID 6725525.