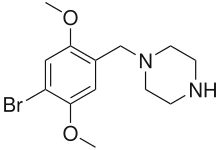
2C-B-BZP
 | |
 | |
| Legal status | |
|---|---|
| Legal status |
|
| Identifiers | |
IUPAC name
| |
| CAS Number | |
| ChemSpider | |
| UNII | |
| CompTox Dashboard (EPA) | |
| Chemical and physical data | |
| Formula | C13H19BrN2O2 |
| Molar mass | 315.211 g·mol−1 |
| 3D model (JSmol) | |
SMILES
| |
InChI
| |
| | |
4-Bromo-2,5-dimethoxy-1-benzylpiperazine (2C-B-BZP) is a psychoactive drug and research chemical of the piperazine chemical class which has been sold as a "designer drug".[1][2] It produces stimulant effects similar to those of benzylpiperazine (BZP).
Chemistry
2C-B-BZP contains a benzylpiperazine base as well as the ring-substitution pattern of the psychedelic phenethylamine 2C-B. 2C-B-BZP is not a phenethylamine itself and does not produce psychedelic effects, as the binding groups are in the wrong position to activate the 5-HT2A receptor, while the phenylpiperazine homologue 2C-B-PP substitutes for DOM in DOM-trained rats with around 1/10th the potency of DOM, but does not substitute for TFMPP.[3]: 867–868
Effects
2C-B-BZP produces stimulant effects which last 3–6 hours. It is also said by several sources to increase the effects of other compounds when combined . Side effects include headaches and nausea, similar to those of other recreationally-used piperazine derivatives.
Legality
2C-B-BZP is unscheduled and uncontrolled in the United States, but possession and sale of 2C-B-BZP could possibly be prosecuted under the Federal Analog Act because of its structural similarities to benzylpiperazine. 2C-B-BZP is illegal to possess, use or sell in Japan where it used to be sold in local smartshops.
See also
References
- ↑ Westphal F, Junge T, Girreser U, Stobbe S, Pérez SB (May 2009). "Structure elucidation of a new designer benzylpiperazine: 4-bromo-2,5-dimethoxybenzylpiperazine". Forensic Science International. 187 (1–3): 87–96. doi:10.1016/j.forsciint.2009.03.003. PMID 19345524.
- ↑ Peters FT, Martinez-Ramirez JA (October 2010). "Analytical toxicology of emerging drugs of abuse". Therapeutic Drug Monitoring. 32 (5): 532–9. doi:10.1097/FTD.0b013e3181f33411. PMID 20814349.
- ↑ Daniel Trachsel; David Lehmann & Christoph Enzensperger (2013). Phenethylamine: Von der Struktur zur Funktion. Nachtschatten Verlag AG. ISBN 978-3-03788-700-4.