Paracetamol
 | |
 | |
| Names | |
|---|---|
| Pronunciation | Paracetamol: /ˌpærəˈsiːtəmɒl/ Acetaminophen: /əˌsiːtəˈmɪnəfɪn/ ( |
| Trade names | Tylenol, Panadol, others[1] |
| Other names | N-acetyl-para-aminophenol (APAP), acetaminophen (USAN US) |
IUPAC name
| |
| Clinical data | |
| Drug class | Analgesics, antipyretics |
| Main uses | Fever, mild pain[2] |
| Side effects | Generally safe at recommended doses[3] |
| WHO AWaRe | UnlinkedWikibase error: ⧼unlinkedwikibase-error-statements-entity-not-set⧽ |
| Pregnancy category | |
| Routes of use | By mouth, through the cheek, rectal, intravenous (IV) |
| Onset of action | Pain relief onset by route: By mouth – 37 minutes[5] Buccal – 15 minutes[5] Intravenous – 8 minutes[5] |
| Defined daily dose | 3 g (by mouth, injection, rectal)[6] |
| External links | |
| AHFS/Drugs.com | Monograph |
| MedlinePlus | a681004 |
| Legal | |
| License data |
|
| Legal status |
|
| Pharmacokinetics | |
| Bioavailability | 63–89%[7]: 73 |
| Protein binding | 10–25%[8] |
| Metabolism | Predominantly in the liver[9] |
| Metabolites | APAP gluc, APAP sulfate, APAP GSH, APAP cys, NAPQI[10] |
| Elimination half-life | 2–2.5 hours[11] |
| Excretion | Urine (85–90%)[9] |
| Chemical and physical data | |
| Formula | C8H9NO2 |
| Molar mass | 151.163 g·mol−1 |
| 3D model (JSmol) | |
| Density | 1.263 g/cm3 |
| Melting point | 169 °C (336 °F) [12][13] |
| Boiling point | 420 °C (788 °F) |
| Solubility in water | |
SMILES
| |
InChI
| |
Paracetamol, also known as acetaminophen, is a medication used to treat pain and fever.[15][16] It is typically used for mild to moderate pain relief.[15] Evidence is mixed for its use to relieve fever in children.[17][18] It is often sold in combination with other medications, such as in many cold medications.[15] Paracetamol is also used for severe pain, such as cancer pain and pain after surgery, in combination with opioid pain medication.[19] It is typically used either by mouth or rectally, but is also available by injection into a vein.[15][20] Effects last between two and four hours.[20]
Paracetamol is generally safe at recommended doses.[3][21] The recommended maximum daily dose for an adult is three to four grams.[22][23][21] Higher doses may lead to toxicity, including liver failure.[15] Serious skin rashes may rarely occur.[15] It appears to be safe during pregnancy and when breastfeeding.[15] In those with liver disease, it may still be used, but in lower doses.[24] It is classified as a mild analgesic.[20] It does not have significant anti-inflammatory activity.[25] How it works is not entirely clear.[25][26][27]
Paracetamol was first made in 1877.[28] It is the most commonly used medication for pain and fever in both the United States and Europe.[29] It is on the World Health Organization's List of Essential Medicines.[30] Paracetamol is available as a generic medication, with brand names including Tylenol and Panadol among others.[31] The wholesale price in the developing world is less than US$0.01 per dose.[32] In the United States, it costs about US$0.04 per dose.[33] In 2017, it was the 25th-most commonly prescribed medication in the United States, with more than 24 million prescriptions.[34][35]
Medical uses
Fever
Paracetamol is used for reducing fever in people of all ages.[15] The World Health Organization recommends that paracetamol be used to treat fever in children only if their temperature is higher than 38.5 °C (101.3 °F).[36] The efficacy of paracetamol by itself in children with fevers has been questioned[37] and a meta-analysis showed that it is less effective than ibuprofen.[38] Paracetamol does not have significant anti-inflammatory effects.[25][26][27]
Pain
Paracetamol is used for the relief of mild to moderate pain.[15] The use of the intravenous form for short-term pain in people in the emergency department is supported by limited evidence.[39] In adults, it appears to be useful for migraines, tension headaches, perineal pain after childbirth, and kidney stone pain.[40]
Osteoarthritis
The American College of Rheumatology recommends paracetamol as one of several treatment options for people with arthritis pain of the hip, hand, or knee that does not improve with exercise and weight loss.[41] A 2015 review, however, found it provided only a small benefit in osteoarthritis.[21]
Paracetamol has relatively little anti-inflammatory activity,[25][26][27] unlike other common analgesics such as the nonsteroidal anti-inflammatory drugs (NSAIDs) aspirin, and ibuprofen, but ibuprofen and paracetamol have similar effects in the treatment of headache. Paracetamol can relieve pain in mild arthritis, but has no effect on the underlying inflammation, redness, and swelling of the joint.[42]
Lower back
Based on a systematic review, paracetamol was recommended by the American College of Physicians and the American Pain Society as a first-line treatment for lower back pain.[43][44][45] The American College of Physicians, as of 2017, noted evidence that it was no different than placebo in the treatment of nonradicular low back pain.[46] Other systematic reviews have also concluded that evidence for its efficacy is lacking.[21][47][48]
Headaches
A joint statement of the German, Austrian, and Swiss headache societies and the German Society of Neurology recommends the use of paracetamol in combination with caffeine as one of several first-line therapies for treatment of tension and migraine headaches.[49] In the treatment of acute migraine, it is superior to placebo, with 39% of people experiencing pain relief at one hour compared with 20% in the control group.[50]
Postoperative
Paracetamol combined with NSAIDs may be more effective for treating postoperative pain than either paracetamol or NSAIDs alone.[51]
Teeth
NSAIDs such as ibuprofen, naproxen, and diclofenac are more effective than paracetamol for controlling dental pain or pain arising from dental procedures; combinations of NSAIDs and paracetamol are more effective than either alone.[52] Paracetamol is particularly useful when NSAIDs are contraindicated due to hypersensitivity or history of gastrointestinal ulceration or bleeding.[53] It can also be used in combination with NSAIDs when these are ineffective in controlling dental pain alone.[54] The Cochrane review of preoperative analgesics for additional pain relief in children and adolescents shows no evidence of benefit in taking paracetamol before dental treatment to help reduce pain after treatment for procedures under local anaesthetic, but the quality of evidence is low.[55]
Combination medications
The efficacy of paracetamol when used in combination with weak opioids (such as codeine) improved for about 50% of people, but with increases in the number experiencing side effects.[56][57] Combination drugs of paracetamol and strong opioids such as morphine improve analgesic effect.[58]
The combination of paracetamol with caffeine is superior to paracetamol alone for the treatment of common pain conditions, including dental pain, post partum pain, and headache.[59]
Patent ductus arteriosus
Paracetamol is used to treat patent ductus arteriosus, a condition that affects newborns when a blood vessel used in developing the lungs fails to close as it normally does, but evidence for the safety and efficacy of paracetamol for this purpose is lacking.[60][61] NSAIDs, particularly indomethacin and ibuprofen, have also been used, but the evidence for them is also not strong.[60] The condition appears to be caused in part by overactive prostaglandin E2 (PGE2), signalling primarily through its EP4 receptor, but possibly also through its EP2 receptor and EP3 receptors.[60]
Dosage
The defined daily dose is 3 g (by mouth) or 3 g (parenteral) or 3 g (rectal)[6] In those who weight more than 50 kg, 1,000 mg can be taken three times per day.[62] For those 30 to 50 kg 500 mg can be used three times per day.[62] Otherwise in children over one month old 15 mg/kg three or four times per day may be used.[62] It may also be given by injection at doses of 1 gram every 6 hours in adults when the medication by mouth is not possible.[2] In those 10 to 50 kg 15 mg/kg may be used, while in those less than 10 kg the dose is 7.5 to 10 mg/kg.[2]
Side effects
Healthy adults taking regular doses up to 4,000 mg a day show little evidence of toxicity.[21] They are more likely to have abnormal liver function tests, but the importance of this is uncertain.[21] There are concerns that continuous use for a few weeks increases systolic blood pressure by about 3 to 4 mmHg; though effects of long term use are unclear.[63]
Liver damage
Acute overdoses of paracetamol can cause potentially fatal liver damage. In 2011, the U.S. Food and Drug Administration (FDA) launched a public-education program to help consumers avoid overdose, warning: "Acetaminophen can cause serious liver damage if more than directed is used."[64][65][66] In a 2011 Safety Warning, the FDA immediately required manufacturers to update labels of all prescription combination acetaminophen products to warn of the potential risk for severe liver injury and required that such combinations contain no more than 325 mg of acetaminophen.[67][68] Overdoses are frequently related to high-dose recreational use of prescription opioids, as these opioids are most often combined with acetaminophen.[69] The overdose risk may be heightened by frequent consumption of alcohol.[16]
Paracetamol toxicity is the foremost cause of acute liver failure in the Western world, and accounts for most drug overdoses in the United States, the United Kingdom, Australia, and New Zealand.[70][71][72][73] According to the FDA, in the United States, "56,000 emergency room visits, 26,000 hospitalizations, and 458 deaths per year [were] related to acetaminophen-associated overdoses during the 1990s. Within these estimates, unintentional acetaminophen overdose accounted for nearly 25% of the emergency department visits, 10% of the hospitalizations, and 25% of the deaths."[74]
Paracetamol is metabolized by the liver and is hepatotoxic; side effects are multiplied when combined with alcoholic drinks, and are very likely in chronic alcoholics or people with liver damage.[75][76] Some studies have suggested the possibility of a moderately increased risk of upper gastrointestinal complications such as stomach bleeding when high doses are taken chronically.[77] Kidney damage is seen in rare cases, most commonly in overdose.[78]
Skin reactions
In 2013, the U.S. Food and Drug Administration (FDA) issued a warning that the drug could cause rare and possibly fatal skin reactions such as Stevens–Johnson syndrome (SJS) and toxic epidermal necrolysis (TEN). Prescription-strength products would be required to carry a warning label about skin reactions, and the FDA urged manufacturers to do the same with over-the-counter products.[79]
Asthma
An association exists between paracetamol use and asthma, but whether this association is causal is still debated as of 2017.[80] Certain evidence suggests that this association likely reflects confounders[81] rather than being truly causal.[82] A 2014 review found that among children, the association disappeared when respiratory infections were taken into account.[83]
As of 2014, the American Academy of Pediatrics and the National Institute for Health and Care Excellence continue to recommend paracetamol for pain and discomfort in children,[84][85][86][87][88][89] but some experts have recommended that paracetamol use by children with asthma or at risk for asthma should be avoided.[90][91]
Other factors
In contrast to aspirin, paracetamol does not prevent blood from clotting (it is not an antiplatelet), thus it may be used in people who have concerns with blood coagulation. Additionally it does not cause gastric irritation.[92] However, paracetamol does not help reduce inflammation, while aspirin does.[93] Compared with ibuprofen—whose side effects may include diarrhea, vomiting and abdominal pain—paracetamol has fewer adverse gastrointestinal effects.[94] Unlike aspirin, paracetamol is generally considered safe for children, as it is not associated with a risk of Reye's syndrome in children with viral illnesses.[95] If taken recreationally with opioids, weak evidence suggests that it may cause hearing loss.[96] Paracetamol use may also inhibit the feeling of empathy for another's pain.[97]
Overdose
In general, the recommended maximum daily dose of paracetamol for healthy adults is three or four grams.[22][23] Higher doses may lead to toxicity.
Untreated paracetamol overdose results in a lengthy, painful illness. Signs and symptoms of paracetamol toxicity may initially be absent or non-specific symptoms. The first symptoms of overdose usually begin several hours after ingestion, with nausea, vomiting, sweating, and pain as acute liver failure starts.[98] People who take overdoses of paracetamol do not fall asleep or lose consciousness, although most people who attempt suicide with paracetamol wrongly believe that they will be rendered unconscious by the drug.[99] The process of dying from an overdose takes from 3–5 days to 4–6 weeks.
Paracetamol hepatotoxicity is by far the most common cause of acute liver failure in both the United States and the United Kingdom.[73][100] Paracetamol overdose results in more calls to poison control centers in the US than overdose of any other pharmacological substance.[101] Toxicity of paracetamol is believed to be due to its quinone metabolite NAPQI.[102]
Untreated overdose can lead to liver failure and death within days. Treatment is aimed at removing the paracetamol from the body and replenishing glutathione.[102] Activated charcoal can be used to decrease absorption of paracetamol if the person comes to the hospital soon after the overdose. While the antidote, acetylcysteine (also called N-acetylcysteine or NAC), acts as a precursor for glutathione, helping the body regenerate enough to prevent or at least decrease the possible damage to the liver; a liver transplant is often required if damage to the liver becomes severe.[70][103] NAC was usually given following a treatment nomogram (one for people with risk factors, and one for those without), but the use of the nomogram is no longer recommended as evidence to support the use of risk factors was poor and inconsistent, and many of the risk factors are imprecise and difficult to determine with sufficient certainty in clinical practice.[104][105] NAC also helps in neutralizing the imidoquinone metabolite of paracetamol.[102] Kidney failure is also a possible side effect.[16]
Until 2004, tablets were available in the UK (brand-name Paradote) that combined paracetamol with an antidote (methionine) to protect the liver in case of an overdose. One theoretical, but rarely if ever used, option in the United States is to request a compounding pharmacy to make a similar drug mix for people who are at risk.
In June 2009, an FDA advisory committee recommended that new restrictions be placed on paracetamol use in the United States to help protect people from the potential toxic effects. The maximum dosage at any given time would be decreased from 1000 mg to 650 mg, while combinations of paracetamol and opioid analgesics would be prohibited. Committee members were particularly concerned by the fact that the then-present maximum dosages of paracetamol had been shown to produce alterations in liver function.[106]
In 2011, the FDA asked manufacturers of prescription combination products containing paracetamol to limit its amount to no more than 325 mg per tablet or capsule and began requiring manufacturers to update the labels of all prescription combination paracetamol products to warn of the potential risk of severe liver damage.[107][68][108][109] Manufacturers had three years to limit the amount of paracetamol in their prescription drug products to 325 mg per dosage unit.[68][109] In November 2011, the Medicines and Healthcare products Regulatory Agency revised UK dosing of liquid paracetamol for children.[110]
Pregnancy
Experimental studies in animals and cohort studies in humans indicate no detectable increase in congenital malformations associated with paracetamol use during pregnancy.[111] Additionally, paracetamol does not affect the closure of the fetal ductus arteriosus as NSAIDs can.[112]
Paracetamol use by the mother during pregnancy is associated with an increased risk of childhood asthma.[113] It is also associated with an increase in ADHD but it is unclear whether the relationship is causal.[114] A 2015 review states that paracetamol remains a first-line recommended medication for pain and fever during pregnancy, despite these concerns.[115]
Cancer
Some studies have found an association between paracetamol and a slight increase in kidney cancer,[116] but no effect on bladder cancer risk.[117]
Pharmacology
Pharmacodynamics
Despite its common use, the mechanism of action of paracetamol is not completely understood. Unlike NSAIDs such as aspirin, paracetamol does not appear to inhibit the function of any cyclooxygenase (COX) enzyme outside the central nervous system, and this appears to be the reason why it is not useful as an anti-inflammatory.[26] It does appear to selectively inhibit COX activities in the brain, which may contribute to its ability to treat fever and pain.[26] This activity does not appear to be direct inhibition by blocking an active site, but rather by reducing COX, which must be oxidized in order to function.[26]
Paracetamol apparently might modulate the endogenous cannabinoid system in the brain through its metabolite, AM404, which appears to inhibit the reuptake of the endogenous cannabinoid/vanilloid anandamide by neurons, making it more available to reduce pain. AM404 also appears to be able to directly activate the TRPV1 (older name: vanilloid receptor), which also inhibits pain signals in the brain.[26]
Pharmacokinetics
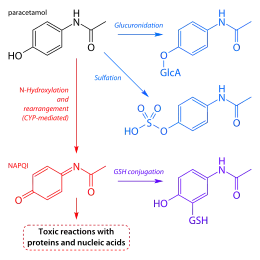
After being taken by mouth, paracetamol is rapidly absorbed by the gastrointestinal (GI) tract (although absorption through the stomach is negligible);[118] its volume of distribution is roughly 50 L.[119] The concentration in serum after a typical dose of paracetamol usually peaks below 30 µg/ml (200 µmol/L).[120] After 4 hours, the concentration is usually less than 10 µg/ml (66 µmol/L).[120]
Paracetamol is metabolized primarily in the liver, into toxic and nontoxic products. Three metabolic pathways are notable:[102]
- Glucuronidation (45–55%),[9] by UGT1A1 and UGT1A6;[117]
- Sulfation (sulfate conjugation) (20–30%)[9] by SULT1A1;[117]
- N-hydroxylation and dehydration, then glutathione conjugation, (less than 15%). The hepatic cytochrome P450 enzyme system metabolises paracetamol (mainly CYP2E1), forming a minor yet significant alkylating metabolite known as NAPQI (N-acetyl-p-benzoquinone imine) (also known as N-acetylimidoquinone).[102][121] NAPQI is then irreversibly conjugated with the sulfhydryl groups of glutathione.[121]
All three pathways yield final products that are inactive, nontoxic, and eventually excreted by the kidneys. In the third pathway, however, the intermediate product NAPQI is toxic. NAPQI is primarily responsible for the toxic effects of paracetamol; this constitutes an example of toxication.[122] Production of NAPQI is due primarily to two isoenzymes of cytochrome P450: CYP2E1[117] and CYP3A4.[122] At usual doses, NAPQI is quickly detoxified by conjugation with glutathione.[102][121]
Chemistry
Chemical properties


Paracetamol consists of a benzene ring core, substituted by one hydroxyl group and the nitrogen atom of an amide group in the para (1,4) pattern.[123] The amide group is acetamide (ethanamide). It is an extensively conjugated system, as one lone pair on the hydroxyl oxygen, the benzene pi cloud, the nitrogen lone pair, the p orbital on the carbonyl carbon, and one lone pair on the carbonyl oxygen are all conjugated. The presence of two activating groups also make the benzene ring highly reactive toward electrophilic aromatic substitution. As the substituents are ortho, para-directing and para with respect to each other, all positions on the ring are more or less equally activated. The conjugation also greatly reduces the basicity of the oxygens and the nitrogen, while making the hydroxyl acidic through delocalisation of charge developed on the phenoxide anion.
Paracetamol is part of the class of drugs known as "aniline analgesics"; it is the only such drug still in use today.[124] It is not considered an NSAID because it does not exhibit significant anti-inflammatory activity (it is a weak COX inhibitor).[27][125] This is despite the evidence that paracetamol and NSAIDs have some similar pharmacological activity.[126]
Synthesis
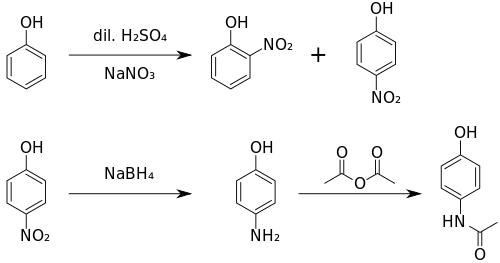
Original (Boots) method
The original method for production involves the nitration of phenol with sodium nitrate gives a mixture of two isomers, from which the wanted 4-nitrophenol (bp 279 °C) can easily be separated by steam distillation. In this electrophilic aromatic substitution reaction, phenol's oxygen is strongly activating, thus the reaction requires only mild conditions as compared to nitration of benzene itself. The nitro group is then reduced to an amine, giving 4-aminophenol. Finally, the amine is acetylated with acetic anhydride.[127] Industrially direct hydrogenation is used, but in the laboratory scale sodium borohydride serves.[128][129]
Green synthesis
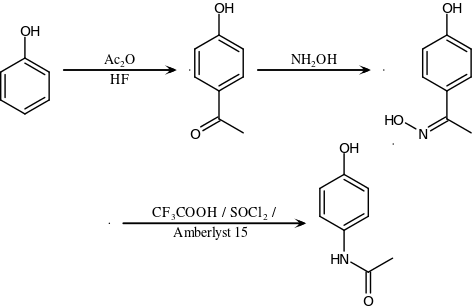
An alternative industrial synthesis developed by Hoechst–Celanese involves direct acylation of phenol with acetic anhydride catalyzed by HF, conversion of the ketone to a ketoxime with hydroxylamine, followed by the acid-catalyzed Beckmann rearrangement to give the amide.[129][130]
Direct synthesis
More recently (2014) a "one-pot" synthesis from hydroquinone has been described before the Royal Society of Chemistry.[131][132] The process may be summarized as follows:
- Hydroquinone, ammonium acetate, and acetic acid were mixed in an argon atmosphere and heated slowly to 230 °C. The mixture was stirred at this temperature for 15 hours. After cooling the acetic acid was evaporated and the precipitate was filtered, washed with water and dried to give paracetamol as a white solid.
The authors go on to claim an 88% yield and 99% purity.
Reactions
4-Aminophenol may be obtained by the amide hydrolysis of paracetamol. 4-Aminophenol prepared this way, and related to the commercially available Metol, has been used as a developer in photography by hobbyists.[133] This reaction is also used to determine paracetamol in urine samples: After hydrolysis with hydrochloric acid, 4-aminophenol reacts in ammonia solution with a phenol derivate, e.g. salicylic acid, to form an indophenol dye under oxidization by air.[134]
History

Acetanilide was the first aniline derivative serendipitously found to possess analgesic as well as antipyretic properties, and was quickly introduced into medical practice under the name of Antifebrin by Cahn & Hepp in 1886.[135] But its unacceptable toxic effects – the most alarming being cyanosis due to methemoglobinemia – prompted the search for less toxic aniline derivatives.[124] Harmon Northrop Morse had already synthesized paracetamol at Johns Hopkins University via the reduction of p-nitrophenol with tin in glacial acetic acid in 1877,[136][137] but it was not until 1887 that clinical pharmacologist Joseph von Mering tried paracetamol on humans.[124] In 1893, von Mering published a paper reporting on the clinical results of paracetamol with phenacetin, another aniline derivative.[138] Von Mering claimed that, unlike phenacetin, paracetamol had a slight tendency to produce methemoglobinemia. Paracetamol was then quickly discarded in favor of phenacetin. The sales of phenacetin established Bayer as a leading pharmaceutical company.[139] Overshadowed in part by aspirin, introduced into medicine by Heinrich Dreser in 1899, phenacetin was popular for many decades, particularly in widely advertised over-the-counter "headache mixtures", usually containing phenacetin, an aminopyrine derivative or aspirin, caffeine, and sometimes a barbiturate.[124]
Paracetamol is the active metabolite of phenacetin and acetanilide, both once popular as analgesics and antipyretics in their own right.[119][140] However, unlike phenacetin, acetanilide and their combinations, paracetamol is not considered carcinogenic at therapeutic doses.[141]
Von Mering's claims remained essentially unchallenged for half a century, until two teams of researchers from the United States analyzed the metabolism of acetanilide and paracetamol.[139] In 1947, David Lester and Leon Greenberg found strong evidence that paracetamol was a major metabolite of acetanilide in human blood, and in a subsequent study they reported that large doses of paracetamol given to albino rats did not cause methemoglobinemia.[142] In three papers published in the September 1948 issue of the Journal of Pharmacology and Experimental Therapeutics, Bernard Brodie, Julius Axelrod and Frederick Flinn confirmed using more specific methods that paracetamol was the major metabolite of acetanilide in human blood, and established that it was just as efficacious an analgesic as its precursor.[143][144][145] They also suggested that methemoglobinemia is produced in humans mainly by another metabolite, phenylhydroxylamine. A follow-up paper by Brodie and Axelrod in 1949 established that phenacetin was also metabolized to paracetamol.[146] This led to a "rediscovery" of paracetamol.[124] It has been suggested that contamination of paracetamol with 4-aminophenol, the substance von Mering synthesised it from, may be the cause for his spurious findings.[139]
Paracetamol was first marketed in the United States in 1950 under the name Triagesic, a combination of paracetamol, aspirin, and caffeine.[137] Reports in 1951 of three users stricken with the blood disease agranulocytosis led to its removal from the marketplace, and it took several years until it became clear that the disease was unconnected.[137] Paracetamol was marketed in 1953 by Sterling-Winthrop Co. as Panadol, available only by prescription, and promoted as preferable to aspirin since it was safe for children and people with ulcers.[137][139][147] In 1955, paracetamol was marketed as Children's Tylenol Elixir by McNeil Laboratories.[148] In 1956, 500 mg tablets of paracetamol went on sale in the United Kingdom under the brand name Panadol, produced by Frederick Stearns & Co, a subsidiary of Sterling Drug Inc. In 1963, paracetamol was added to the British Pharmacopoeia, and has gained popularity since then as an analgesic agent with few side-effects and little interaction with other pharmaceutical agents.[137] Concerns about paracetamol's safety delayed its widespread acceptance until the 1970s, but in the 1980s paracetamol sales exceeded those of aspirin in many countries, including the United Kingdom. This was accompanied by the commercial demise of phenacetin, blamed as the cause of analgesic nephropathy and hematological toxicity.[124] In 1988, Sterling Winthrop was acquired by Eastman Kodak which sold the over the counter drug rights to SmithKline Beecham in 1994.[149]
Available without a prescription since 1959,[150] it has since become a common household drug.[151] Patents on paracetamol have long expired, and generic versions of the drug are widely available.[1][152]
Society and culture
Naming
Acetaminophen is the name generally used in the United States (United States Adopted Name), Japan (Japanese Accepted Name), Canada,[153] Venezuela, Colombia, and Iran; paracetamol is used in international venues (International Nonproprietary Name, Australian Approved Name, British Approved Name).[153][154][155] In some contexts, such as on prescription bottles of painkillers that incorporate this medicine, it is simply abbreviated as APAP, for acetyl-para-aminophenol.
Both acetaminophen and paracetamol come from a chemical name for the compound: para-acetylaminophenol and para-acetylaminophenol.
Cost
The wholesale price in the developing world is less than US$0.01 per dose.[32] In the United States, it costs about US$0.04 per dose.[33]
In Europe, prices differ from country to country; low-cost prices for 10 doses could reach €0.54 in Portugal, €0.91 in France and €1.97 in Germany.[156]
.svg.png.webp) Acetaminophen costs (US)
Acetaminophen costs (US).svg.png.webp) Acetaminophen prescriptions (US)
Acetaminophen prescriptions (US)
Available forms
Paracetamol is available in tablet, capsule, liquid suspension, suppository, intravenous, intramuscular and effervescent forms.[157][158] Intravenous acetaminophen is sold under the brand name Ofirmev in the United States.[159] It costs about 50 USD per 1,000 mg dose as of 2021.[160]
In some formulations, paracetamol is combined with the opiate codeine, sometimes referred to as co-codamol (BAN) and Panadeine in Australia. In the U.S., this combination is available only by prescription,[161] while the lowest-strength preparation is over the counter in Canada, and in other countries other strengths may be available over the counter. Paracetamol is also combined with other opioids such as dihydrocodeine,[162] referred to as co-dydramol (British Approved Name (BAN)), oxycodone[163] or hydrocodone.[164] Another very commonly used analgesic combination includes paracetamol in combination with propoxyphene napsylate.[165] A combination of paracetamol, codeine, and the calmative doxylamine succinate is also available.[166] The efficacy of paracetamol/codeine combinations has been questioned by research from 2010.[58]
Paracetamol is commonly used in multi-ingredient preparations for migraine headache, typically including butalbital and paracetamol with or without caffeine, and sometimes containing codeine.
Paracetamol is sometimes combined with phenylephrine hydrochloride.[167] Sometimes a third active ingredient, such as ascorbic acid,[167][168] caffeine,[169][170] chlorpheniramine maleate,[171] or guaifenesin[172][173][174] is added to this combination.
When marketed in combination with diphenhydramine hydrochloride, it is frequently given the label "PM" and is meant as a sleep aid. Diphenhydramine hydrochloride is known to have hypnotic effects and is non-habit forming. Unfortunately, it has been implicated in the occasional development of restless leg syndrome.[175]
 Tylenol 500 mg capsules
Tylenol 500 mg capsules Panadol 500 mg tablets
Panadol 500 mg tablets For comparison: The pure drug is a colourless crystalline powder.
For comparison: The pure drug is a colourless crystalline powder.
Controversy
In September 2013, an episode of This American Life titled "Use Only as Directed"[176] highlighted deaths from paracetamol overdose. This report was followed by two reports by ProPublica alleging that the "FDA has long been aware of studies showing the risks of acetaminophen. So has the maker of Tylenol, McNeil Consumer Healthcare, a division of Johnson & Johnson"[177] and "McNeil, the maker of Tylenol, ... has repeatedly opposed safety warnings, dosage restrictions and other measures meant to safeguard users of the drug."[178]
A report prepared by an internal FDA working group describes a history of FDA initiatives designed to educate consumers about the risk of paracetamol overdose and notes that one challenge to the Agency has been "identifying the appropriate message about the relative safety of acetaminophen, especially compared to other OTC pain relievers (e.g., aspirin and other NSAIDs)". The report notes that "Chronic use of NSAIDs is also associated with significant morbidity and mortality. NSAID gastrointestinal risk is substantial, with deaths and hospitalization estimated in one publication as 3200 and 32,000 per year respectively. Possible cardiovascular toxicity with chronic NSAID use has been a major discussion recently", finally noting that "The goal of the educational efforts is not to decrease appropriate acetaminophen use or encourage substitution of NSAID use, but rather to educate consumers so that they can avoid unnecessary health risks."[179]
Veterinary use
Cats
Paracetamol is extremely toxic to cats, which lack the necessary UGT1A6 enzyme to break it down safely. Initial symptoms include vomiting, salivation, and discoloration of the tongue and gums.
Unlike an overdose in humans, liver damage is rarely the cause of death; instead, methemoglobin formation and the production of Heinz bodies in red blood cells inhibit oxygen transport by the blood, causing asphyxiation (methemoglobemia and hemolytic anemia).[180]
Treatment with N-acetylcysteine,[181] methylene blue or both is sometimes effective after the ingestion of small doses of paracetamol.
Dogs
Although paracetamol is believed to have no significant anti-inflammatory activity, it has been reported to be as effective as aspirin in the treatment of musculoskeletal pain in dogs.[182]
A paracetamol-codeine product (brand name Pardale-V)[183] licensed for use in dogs is available for purchase under supervision of a vet, pharmacist or other qualified person.[183] It should be administered to dogs only on veterinary advice and with extreme caution.[183]
The main effect of toxicity in dogs is liver damage, and GI ulceration has been reported.[181][184][185][186] N-acetylcysteine treatment is efficacious in dogs when administered within two hours of paracetamol ingestion.[181][182]
Snakes
Paracetamol is lethal to snakes, and has been suggested as a chemical control program for the invasive brown tree snake (Boiga irregularis) in Guam.[187][188] Doses of 80 mg are inserted into dead mice that are scattered by helicopter.[189]
References
- 1 2 International Drug Names
- 1 2 3 "PARACETAMOL = ACETAMINOPHEN injectable - Essential drugs". medicalguidelines.msf.org. Archived from the original on 28 August 2021. Retrieved 4 September 2020.
- 1 2 Russell FM, Shann F, Curtis N, Mulholland K (2003). "Evidence on the use of paracetamol in febrile children". Bulletin of the World Health Organization. 81 (5): 367–72. hdl:10665/268935. ISSN 0042-9686. PMC 2572451. PMID 12856055.
- 1 2 "Acetaminophen Use During Pregnancy". Drugs.com. 14 June 2019. Archived from the original on 9 March 2020. Retrieved 25 February 2020.
- 1 2 3 Pickering G, Macian N, Libert F, Cardot JM, Coissard S, Perovitch P, Maury M, Dubray C (September 2014). "Buccal acetaminophen provides fast analgesia: two randomized clinical trials in healthy volunteers". Drug Des. Devel. Ther. 8: 1621–1627. doi:10.2147/DDDT.S63476. PMC 4189711. PMID 25302017.
bAPAP has a faster time of antinociception onset (15 minutes, P<0.01) and greater antinociception at 50 minutes (P<0.01, CT1) and 30 minutes (P<0.01, CT2) than ivAPAP and sAPAP. All routes are similar after 50 minutes. ... In postoperative conditions for acute pain of mild to moderate intensity, the quickest reported time to onset of analgesia with APAP is 8 minutes9 for the iv route and 37 minutes6 for the oral route.
- 1 2 "WHOCC - ATC/DDD Index". www.whocc.no. Archived from the original on 2 October 2020. Retrieved 22 September 2020.
- ↑ Working Group of the Australian and New Zealand College of Anaesthetists and Faculty of Pain Medicine (2015). Schug SA, Palmer GM, Scott DA, Halliwell R, Trinca J (eds.). Acute Pain Management: Scientific Evidence (4th ed.). Melbourne: Australian and New Zealand College of Anaesthetists (ANZCA), Faculty of Pain Medicine (FPM). ISBN 978-0-9873236-7-5. Archived from the original (PDF) on 31 July 2019. Retrieved 28 October 2019.
- ↑ "Tylenol, Tylenol Infants' Drops (acetaminophen) dosing, indications, interactions, adverse effects, and more". Medscape Reference. Archived from the original on 14 April 2014. Retrieved 10 May 2014.
- 1 2 3 4 "Codapane Forte Paracetamol and codeine phosphate product information" (PDF). TGA eBusiness Services. Alphapharm Pty Limited. 29 April 2013. Archived from the original on 6 February 2016. Retrieved 10 May 2014.
- ↑ "Acetaminophen Pathway (therapeutic doses), Pharmacokinetics". Archived from the original on 4 March 2016. Retrieved 13 January 2016.
- ↑ Wong A, Graudins A (2017). "Risk prediction of hepatotoxicity in paracetamol poisoning". Clin Toxicol (Phila). 55: 879–892. doi:10.1080/15563650.2017.1317349. PMID 28447858.
{{cite journal}}: CS1 maint: uses authors parameter (link) - ↑ Karthikeyan M, Glen RC, Bender A (2005). "General Melting Point Prediction Based on a Diverse Compound Data Set and Artificial Neural Networks". Journal of Chemical Information and Modeling. 45 (3): 581–590. doi:10.1021/ci0500132. PMID 15921448.
- ↑ "melting point data for paracetamol". Lxsrv7.oru.edu. Archived from the original on 30 June 2012. Retrieved 19 March 2011.
- 1 2 3 4 5 Granberg RA, Rasmuson AC (1999). "Solubility of paracetamol in pure solvents". Journal of Chemical & Engineering Data. 44 (6): 1391–95. doi:10.1021/je990124v.
- 1 2 3 4 5 6 7 8 9 "Acetaminophen". The American Society of Health-System Pharmacists. Archived from the original on 5 June 2016. Retrieved 16 September 2016.
- 1 2 3 Lee, WM (December 2017). "Acetaminophen (APAP) hepatotoxicity-Isn't it time for APAP to go away?". Journal of Hepatology. 67 (6): 1324–1331. doi:10.1016/j.jhep.2017.07.005. PMC 5696016. PMID 28734939.
- ↑ Meremikwu M, Oyo-Ita A (2002). "Paracetamol for treating fever in children". The Cochrane Database of Systematic Reviews (2): CD003676. doi:10.1002/14651858.CD003676. PMC 6532671. PMID 12076499.
- ↑ de Martino M, Chiarugi A (2015). "Recent Advances in Pediatric Use of Oral Paracetamol in Fever and Pain Management". Pain and Therapy. 4 (2): 149–168. doi:10.1007/s40122-015-0040-z. PMC 4676765. PMID 26518691.
- ↑ Scottish Intercollegiate Guidelines Network (SIGN) (2008). "6.1 and 7.1.1" (PDF). Guideline 106: Control of pain in adults with cancer. Scotland: National Health Service (NHS). ISBN 9781905813384. Archived (PDF) from the original on 20 December 2010.
- 1 2 3 Hochhauser, Daniel (2014). Cancer and its Management. John Wiley & Sons. p. 119. ISBN 9781118468715. Archived from the original on 8 September 2017.
- 1 2 3 4 5 6 Machado GC, Maher CG, Ferreira PH, Pinheiro MB, Lin CW, Day RO, McLachlan AJ, Ferreira ML (31 March 2015). "Efficacy and safety of paracetamol for spinal pain and osteoarthritis: systematic review and meta-analysis of randomised placebo controlled trials". BMJ (Clinical Research Ed.). 350: h1225. doi:10.1136/bmj.h1225. PMC 4381278. PMID 25828856.
- 1 2 "Paracetamol for adults: painkiller to treat aches, pains and fever". National Health Service. Archived from the original on 22 August 2017. Retrieved 22 August 2017.
- 1 2 "What are the recommended maximum daily dosages of acetaminophen in adults and children?". Medscape. Archived from the original on 21 December 2018. Retrieved 19 December 2018.
- ↑ Lewis JH, Stine JG (June 2013). "Review article: prescribing medications in patients with cirrhosis - a practical guide". Alimentary Pharmacology & Therapeutics. 37 (12): 1132–56. doi:10.1111/apt.12324. PMID 23638982.
- 1 2 3 4 McKay, Gerard A.; Walters, Matthew R. (2013). "Non-Opioid Analgesics". Lecture Notes Clinical Pharmacology and Therapeutics (9th ed.). Hoboken: Wiley. ISBN 9781118344897. Archived from the original on 8 September 2017. Retrieved 5 August 2020.
- 1 2 3 4 5 6 7 Ghanem CI, Pérez MJ, Manautou JE, Mottino AD (July 2016). "Acetaminophen from liver to brain: New insights into drug pharmacological action and toxicity". Pharmacological Research. 109: 119–31. doi:10.1016/j.phrs.2016.02.020. PMC 4912877. PMID 26921661.
- 1 2 3 4 Viswanathan AN, Feskanich D, Schernhammer ES, Hankinson SE (2008). "Aspirin, NSAID, and Acetaminophen Use and the Risk of Endometrial Cancer". Cancer Research. 68 (7): 2507–2513. doi:10.1158/0008-5472.CAN-07-6257. PMC 2857531. PMID 18381460.
- ↑ Mangus, Brent C.; Miller, Michael G. (2005). Pharmacology application in athletic training. Philadelphia, Pennsylvania: F.A. Davis. p. 39. ISBN 9780803620278. Archived from the original on 8 September 2017. Retrieved 5 August 2020.
- ↑ Aghababian, Richard V. (22 October 2010). Essentials of emergency medicine. Jones & Bartlett Publishers. p. 814. ISBN 978-1-4496-1846-9. Archived from the original on 17 August 2016.
- ↑ World Health Organization (2019). World Health Organization model list of essential medicines: 21st list 2019. Geneva: World Health Organization. hdl:10665/325771. WHO/MVP/EMP/IAU/2019.06. License: CC BY-NC-SA 3.0 IGO.
- ↑ Hamilton, Richard J. (2013). Tarascon pocket pharmacopoeia : 2013 classic shirt-pocket edition (27th ed.). Burlington, Massachusetts: Jones & Bartlett Learning. p. 12. ISBN 9781449665869. Archived from the original on 8 September 2017.
- 1 2 "Paracetamol". Archived from the original on 22 January 2018. Retrieved 11 January 2016.
- 1 2 "Acetaminophen prices, coupons and patient assistance programs". Archived from the original on 16 February 2016. Retrieved 19 February 2016.
- ↑ "The Top 300 of 2020". ClinCalc. Archived from the original on 12 February 2021. Retrieved 25 February 2020.
- ↑ "Acetaminophen Drug Usage Statistics". ClinCalc. Archived from the original on 12 April 2020. Retrieved 11 April 2020.
- ↑ "Baby paracetamol asthma concern". BBC News Online. 19 September 2008. Archived from the original on 20 September 2008. Retrieved 19 September 2008.
- ↑ Meremikwu M, Oyo-Ita A (2002). "Paracetamol for treating fever in children". Cochrane Database Syst Rev (2): CD003676. doi:10.1002/14651858.CD003676. PMC 6532671. PMID 12076499.
- ↑ Perrott DA, Piira T, Goodenough B, Champion GD (2004). "Efficacy and safety of acetaminophen vs ibuprofen for treating children's pain or fever: a meta-analysis". Arch Pediatr Adolesc Med. 158 (6): 521–6. doi:10.1001/archpedi.158.6.521. PMID 15184213.
- ↑ Sin B, Wai M, Tatunchak T, Motov SM (29 January 2016). "The use of intravenous acetaminophen for acute pain in the emergency department". Academic Emergency Medicine. 23 (5): 543–53. doi:10.1111/acem.12921. PMID 26824905.
- ↑ Saragiotto, BT; Abdel Shaheed, C; Maher, CG (31 December 2019). "Paracetamol for pain in adults". BMJ (Clinical research ed.). 367: l6693. doi:10.1136/bmj.l6693. PMID 31892511.
- ↑ Hochberg MC, Altman RD, April KT, Benkhalti M, Guyatt G, McGowan J, et al. (April 2012). "American College of Rheumatology 2012 recommendations for the use of nonpharmacologic and pharmacologic therapies in osteoarthritis of the hand, hip, and knee". Arthritis Care Res (Hoboken). 64 (4): 465–74. doi:10.1002/acr.21596. PMID 22563589. Archived from the original on 15 November 2019. Retrieved 5 August 2020.
- ↑ "Paracetamol". Arthritis Research UK. Archived from the original on 17 October 2013. Retrieved 16 October 2013.
- ↑ "National Guideline Clearinghouse | Expert Commentaries: Diagnosis and Treatment of Low Back Pain: A Joint Clinical Practice Guideline from the American College of Physicians and the American Pain Society. What's New? What's Different?". Archived from the original on 14 September 2014. Retrieved 14 September 2014.
- ↑ Chou R, Qaseem A, Snow V, Casey D, Cross JT Jr, Shekelle P, et al. (2 October 2007). "Diagnosis and Treatment of Low Back Pain: A Joint Clinical Practice Guideline from the American College of Physicians and the American Pain Society". Annals of Internal Medicine. 147 (7): 478–91. doi:10.7326/0003-4819-147-7-200710020-00006. ISSN 0003-4819. PMID 17909209.
- ↑ Chou R, Huffman LH, American Pain Society, American College of Physicians (2 October 2007). "Medications for Acute and Chronic Low Back Pain: A Review of the Evidence for an American Pain Society/American College of Physicians Clinical Practice Guideline". Annals of Internal Medicine. 147 (7): 505–14. doi:10.7326/0003-4819-147-7-200710020-00008. ISSN 0003-4819. PMID 17909211.
- ↑ Qaseem A, Wilt TJ, McLean RM, Forciea MA (April 2017). "Noninvasive Treatments for Acute, Subacute, and Chronic Low Back Pain: A Clinical Practice Guideline From the American College of Physicians". Ann. Intern. Med. 166 (7): 514–530. doi:10.7326/M16-2367. PMID 28192789.
- ↑ Saragiotto BT, Machado GC, Ferreira ML, Pinheiro MB, Abdel Shaheed C, Maher CG (June 2016). "Paracetamol for low back pain". Cochrane Database Syst Rev. 6 (6): CD012230. doi:10.1002/14651858.CD012230. PMC 6353046. PMID 27271789.
- ↑ Machado GC, Maher CG, Ferreira PH, Pinheiro MB, Lin CW, Day RO, McLachlan AJ, Ferreira ML (March 2015). "Efficacy and safety of paracetamol for spinal pain and osteoarthritis: systematic review and meta-analysis of randomised placebo controlled trials". BMJ. 350: h1225. doi:10.1136/bmj.h1225. PMC 4381278. PMID 25828856.
- ↑ Haag G, Diener HC, May A, Meyer C, Morck H, Straube A, et al. (April 2011). "Self-medication of migraine and tension-type headache: summary of the evidence-based recommendations of the Deutsche Migräne und Kopfschmerzgesellschaft (DMKG), the Deutsche Gesellschaft für Neurologie (DGN), the Österreichische Kopfschmerzgesellschaft (ÖKSG) and the Schweizerische Kopfwehgesellschaft (SKG)". J Headache Pain. 12 (2): 201–17. doi:10.1007/s10194-010-0266-4. PMC 3075399. PMID 21181425.
- ↑ Derry S, Moore RA (2013). "Paracetamol (acetaminophen) with or without an antiemetic for acute migraine headaches in adults". Cochrane Database Syst Rev. 4 (4): CD008040. doi:10.1002/14651858.CD008040.pub3. PMC 4161111. PMID 23633349.
- ↑ Ong CK, Seymour RA, Lirk P, Merry AF (1 April 2010). "Combining paracetamol (acetaminophen) with nonsteroidal antiinflammatory drugs: a qualitative systematic review of analgesic efficacy for acute postoperative pain". Anesthesia and Analgesia. 110 (4): 1170–9. doi:10.1213/ANE.0b013e3181cf9281. PMID 20142348.
- ↑ Moore RA, Derry C (January 2013). "Efficacy of OTC analgesics". International Journal of Clinical Practice. Supplement. 67 (178): 21–5. doi:10.1111/ijcp.12054. PMID 23163544.
- ↑ "Relieving dental pain". American Dental Association. December 2016. Archived from the original on 17 November 2016.
- ↑ Bailey E, Worthington H, Coulthard P (April 2014). "Ibuprofen and/or paracetamol (acetaminophen) for pain relief after surgical removal of lower wisdom teeth, a Cochrane systematic review". British Dental Journal. 216 (8): 451–5. doi:10.1038/sj.bdj.2014.330. PMID 24762895.
- ↑ Ashley PF, Parekh S, Moles DR, Anand P, MacDonald LC (8 August 2016). "Preoperative analgesics for additional pain relief in children and adolescents having dental treatment" (PDF). The Cochrane Database of Systematic Reviews (8): CD008392. doi:10.1002/14651858.CD008392.pub3. PMID 27501304. Archived (PDF) from the original on 18 July 2018. Retrieved 5 August 2020.
- ↑ de Craen AJ, Di Giulio G, Lampe-Schoenmaeckers JE, Kessels AG, Kleijnen J (1996). "Analgesic efficacy and safety of paracetamol-codeine combinations versus paracetamol alone: a systematic review". BMJ. 313 (7053): 321–324. doi:10.1136/bmj.313.7053.321. PMC 2351742. PMID 8760737.
- ↑ Toms L, Derry S, Moore RA, McQuay HJ (January 2009). "Single dose oral paracetamol (acetaminophen) with codeine for postoperative pain in adults". Cochrane Database of Systematic Reviews (1): CD001547. doi:10.1002/14651858.CD001547.pub2. PMC 4171965. PMID 19160199.
- 1 2 Murnion BP (August 2010). "Combination analgesics in adults". Australian Prescriber. 33 (4): 113–5. doi:10.18773/austprescr.2010.056.
- ↑ Derry CJ, Derry S, Moore RA (2012). "Caffeine as an analgesic adjuvant for acute pain in adults". Cochrane Database Syst Rev. 3 (3): CD009281. doi:10.1002/14651858.CD009281.pub2. PMID 22419343.
- 1 2 3 Sivanandan S, Agarwal R (2016). "Pharmacological Closure of Patent Ductus Arteriosus: Selecting the Agent and Route of Administration". Paediatric Drugs. 18 (2): 123–38. doi:10.1007/s40272-016-0165-5. PMID 26951240.
- ↑ Sallmon H, Koehne P, Hansmann G (2016). "Recent Advances in the Treatment of Preterm Newborn Infants with Patent Ductus Arteriosus". Clinics in Perinatology. 43 (1): 113–29. doi:10.1016/j.clp.2015.11.008. PMID 26876125.
- 1 2 3 "PARACETAMOL = ACETAMINOPHEN oral". Archived from the original on 29 August 2021. Retrieved 23 August 2020.
- ↑ Ton, Joey (1 May 2023). "#339 Is acetaminophen under pressure?". CFPCLearn. Archived from the original on 1 July 2023. Retrieved 13 June 2023.
- ↑ "Acetaminophen Information". U.S. Food and Drug Administration (FDA). 14 November 2017. Archived from the original on 28 October 2019. Retrieved 27 October 2019.
 This article incorporates text from this source, which is in the public domain.
This article incorporates text from this source, which is in the public domain. - ↑ "Safe Use Initiative: Acetaminophen Toxicity". U.S. Food and Drug Administration (FDA). 1 August 2011. Archived from the original on 1 September 2011. Retrieved 23 February 2014.
 This article incorporates text from this source, which is in the public domain.
This article incorporates text from this source, which is in the public domain. - ↑ "Using Acetaminophen and Nonsteroidal Anti-inflammatory Drugs Safely". U.S. Food and Drug Administration (FDA). 26 February 2018. Archived from the original on 28 October 2019. Retrieved 27 October 2019.
 This article incorporates text from this source, which is in the public domain.
This article incorporates text from this source, which is in the public domain. - ↑ "FDA limits acetaminophen in prescription combination products; requires liver toxicity warnings". U.S. Food and Drug Administration (FDA) (Press release). 15 January 2011. Archived from the original on 15 January 2011. Retrieved 23 February 2014.
 This article incorporates text from this source, which is in the public domain.
This article incorporates text from this source, which is in the public domain. - 1 2 3 "FDA Drug Safety Communication: Prescription Acetaminophen Products to be Limited to 325 mg Per Dosage Unit; Boxed Warning Will Highlight Potential for Severe Liver Failure". U.S. Food and Drug Administration (FDA). 13 January 2011. Archived from the original on 18 January 2011. Retrieved 13 January 2011.
 This article incorporates text from this source, which is in the public domain.
This article incorporates text from this source, which is in the public domain. - ↑ Yan, Holly (16 January 2014). "FDA: Acetaminophen doses over 325 mg may lead to liver damage". CNN. Archived from the original on 16 February 2014. Retrieved 18 February 2014.
- 1 2 Daly FF, Fountain JS, Murray L, Graudins A, Buckley NA (2008). "Guidelines for the management of paracetamol poisoning in Australia and New Zealand—explanation and elaboration. A consensus statement from clinical toxicologists consulting to the Australasian poisons information centres". Med J Aust. 188 (5): 296–301. doi:10.5694/j.1326-5377.2008.tb01625.x. PMID 18312195. Archived from the original on 23 July 2008.
- ↑ Khashab M, Tector AJ, Kwo PY (2007). "Epidemiology of acute liver failure". Curr Gastroenterol Rep. 9 (1): 66–73. doi:10.1007/s11894-008-0023-x. PMID 17335680.
- ↑ Hawkins LC, Edwards JN, Dargan PI (2007). "Impact of restricting paracetamol pack sizes on paracetamol poisoning in the United Kingdom: a review of the literature". Drug Saf. 30 (6): 465–79. doi:10.2165/00002018-200730060-00002. PMID 17536874.
- 1 2 Larson AM, Polson J, Fontana RJ, Davern TJ, Lalani E, Hynan LS, et al. (2005). "Acetaminophen-induced acute liver failure: results of a United States multicenter, prospective study". Hepatology. 42 (6): 1364–72. doi:10.1002/hep.20948. PMID 16317692.
- ↑ U.S. Food and Drug Administration (FDA) Date Posted 14 January 2011. Prescription Drug Products Containing Acetaminophen: Actions to Reduce Liver Injury from Unintentional Overdose Regulations.gov at the Wayback Machine (archived 25 September 2012) Retrieved 23 February 2014
 This article incorporates text from this source, which is in the public domain.
This article incorporates text from this source, which is in the public domain. - ↑ Hughes, John (2008). Pain Management: From Basics to Clinical Practice. Elsevier Health Sciences. ISBN 9780443103360.
- ↑ Dukes MN, Aronson JK (2000). Meyler's Side Effects of Drugs, Vol XIV. Elsevier. ISBN 9780444500939.
- ↑ García Rodríguez LA, Hernández-Díaz S (15 December 2000). "The risk of upper gastrointestinal complications associated with nonsteroidal anti-inflammatory drugs, glucocorticoids, acetaminophen, and combinations of these agents". Arthritis Research & Therapy. 3 (2): 98–101. doi:10.1186/ar146. PMC 128885. PMID 11178116.
- ↑ "Painkillers 'cause kidney damage'". BBC News Online. 23 November 2003. Archived from the original on 26 September 2010. Retrieved 27 March 2010.
- ↑ "FDA Drug Safety Communication: FDA warns of rare but serious skin reactions with the pain reliever/fever reducer acetaminophen". U.S. Food and Drug Administration (FDA). 1 August 2013. Archived from the original on 28 October 2019. Retrieved 27 October 2019.
 This article incorporates text from this source, which is in the public domain.
This article incorporates text from this source, which is in the public domain. - ↑ Lourido-Cebreiro T, Salgado FJ, Valdes L, Gonzalez-Barcala FJ (January 2017). "The association between paracetamol and asthma is still under debate". The Journal of Asthma (Review). 54 (1): 32–8. doi:10.1080/02770903.2016.1194431. PMID 27575940.
- ↑ Henderson AJ, Shaheen SO (March 2013). "Acetaminophen and asthma". Paediatric Respiratory Reviews. 14 (1): 9–15, quiz 16. doi:10.1016/j.prrv.2012.04.004. PMID 23347656.
- ↑ Heintze K, Petersen KU (June 2013). "The case of drug causation of childhood asthma: antibiotics and paracetamol". European Journal of Clinical Pharmacology. 69 (6): 1197–209. doi:10.1007/s00228-012-1463-7. PMC 3651816. PMID 23292157.
- ↑ Cheelo M, Lodge CJ, Dharmage SC, Simpson JA, Matheson M, Heinrich J, Lowe AJ (26 November 2014). "Paracetamol exposure in pregnancy and early childhood and development of childhood asthma: a systematic review and meta-analysis". Archives of Disease in Childhood. 100 (1): 81–9. doi:10.1136/archdischild-2012-303043. PMID 25429049.
- ↑ "Feverish illness in children: Assessment and initial management in children younger than 5 years". NICE clinical guidelines. UK National Institute for Health and Care Excellence. May 2013. Archived from the original on 6 March 2014. Retrieved 25 February 2014.
- ↑ "Common over-the-counter medications". Healthychildren.org. American Academy of Pediatrics. 10 July 2013. Archived from the original on 27 February 2014. Retrieved 23 February 2014.
- ↑ Heintze K, Petersen KU (June 2013). "The case of drug causation of childhood asthma: antibiotics and paracetamol". European Journal of Clinical Pharmacology. 69 (6): 1197–209. doi:10.1007/s00228-012-1463-7. PMC 3651816. PMID 23292157.
- ↑ "Link between Calpol and asthma 'not proven'". NHS Choices. UK National Health Service. 16 September 2013. Archived from the original on 25 February 2014. Retrieved 23 February 2014.
- ↑ Section on Clinical Pharmacology and Therapeutics, Committee on Drugs, Sullivan JE, Farrar HC (March 2011). "Fever and antipyretic use in children". Pediatrics. 127 (3): 580–7. doi:10.1542/peds.2010-3852. PMID 21357332.
- ↑ CHMP Pharmacovigilance Working Party (24 February 2011). Pharmacovigilance Working Party (PhVWP) February 2011 plenary meeting (PDF) (Report). European Medicines Agency & Heads of Medicines Agencies. pp. 6–7. Archived (PDF) from the original on 8 November 2013.
- ↑ Martinez-Gimeno A, García-Marcos L (April 2013). "The association between acetaminophen and asthma: should its pediatric use be banned?". Expert Review of Respiratory Medicine. 7 (2): 113–22. doi:10.1586/ers.13.8. PMID 23547988. Archived from the original on 9 May 2013.
- ↑ McBride, JT (December 2011). "The association of acetaminophen and asthma prevalence and severity". Pediatrics. 128 (6): 1181–5. doi:10.1542/peds.2011-1106. PMID 22065272.
- ↑ Sarg, Michael; Ann D Gross; Altman, Roberta (2007). The Cancer Dictionary. Infobase Publishing. ISBN 9780816064113.
- ↑ Neuss, G (2007). Chemistry: Course Companion. Oxford University Press. ISBN 978-0-19-915146-2.
- ↑ Ebrahimi, Sedigheh; Esfahani, Soheil Ashkani; Ghaffarian, Hamid Reza; Khoshneviszade, Mahsima (2010). "Comparison of efficacy and safety of acetaminophen and ibuprofen administration as single dose to reduce fever in children". Iranian Journal of Pediatrics. 20 (4): 500–501. Archived from the original on 9 July 2012.
- ↑ Lesko SM, Mitchell AA (1999). "The safety of acetaminophen and ibuprofen among children younger than two years old". Pediatrics. 104 (4): e39. doi:10.1542/peds.104.4.e39. PMID 10506264.
- ↑ Yorgason JG, Luxford W, Kalinec F (December 2011). "In vitro and in vivo models of drug ototoxicity: studying the mechanisms of a clinical problem". Expert Opinion on Drug Metabolism & Toxicology. 7 (12): 1521–34. doi:10.1517/17425255.2011.614231. PMID 21999330.
- ↑ Mischkowski D, Crocker J, Way BM (September 2016). "From painkiller to empathy killer: acetaminophen (paracetamol) reduces empathy for pain". Social Cognitive and Affective Neuroscience. 11 (9): 1345–53. doi:10.1093/scan/nsw057. PMC 5015806. PMID 27217114.
- ↑ Rumack B, Matthew H (1975). "Acetaminophen poisoning and toxicity". Pediatrics. 55 (6): 871–76. PMID 1134886.
- ↑ "Paracetamol". University of Oxford Centre for Suicide Research. 25 March 2013. Archived from the original on 20 March 2013. Retrieved 20 April 2013.
- ↑ Ryder SD, Beckingham IJ (2001). "ABC of diseases of liver, pancreas, and biliary system. Other causes of parenchymal liver disease". BMJ. 322 (7281): 290–92. doi:10.1136/bmj.322.7281.290. PMC 1119531. PMID 11157536.
- ↑ Lee WM (2004). "Acetaminophen and the U.S. Acute Liver Failure Study Group: lowering the risks of hepatic failure". Hepatology. 40 (1): 6–9. doi:10.1002/hep.20293. PMID 15239078.
- 1 2 3 4 5 6 Mehta, Sweety (25 August 2012). "Metabolism of Paracetamol (Acetaminophen), Acetanilide and Phenacetin". PharmaXChange.info. Archived from the original on 28 October 2019. Retrieved 27 October 2019.
- ↑ "Highlights of Prescribing Information" (PDF). Acetadote. Archived from the original (PDF) on 22 February 2014. Retrieved 10 February 2014.
- ↑ "Paracetamol overdose: new guidance on treatment with intravenous acetylcysteine". Drug Safety Update. 6 (2): A1. September 2012. Archived from the original on 27 October 2012.
- ↑ "Treating paracetamol overdose with intravenous acetylcysteine: new guidance". Medicines and Healthcare products Regulatory Agency (MHRA). 11 December 2014. Archived from the original on 28 October 2019. Retrieved 27 October 2019.
- ↑ "FDA May Restrict Acetaminophen". Webmd. 1 July 2009. Archived from the original on 21 March 2011. Retrieved 19 March 2011.
- ↑ "FDA limits acetaminophen in prescription combination products; requires liver toxicity warnings" (Press release). U.S. Food and Drug Administration (FDA). 13 January 2011. Archived from the original on 15 January 2011. Retrieved 13 January 2011.
 This article incorporates text from this source, which is in the public domain.
This article incorporates text from this source, which is in the public domain. - ↑ Perrone, Matthew (13 January 2011). "FDA orders lowering pain reliever in Vicodin". The Boston Globe. Associated Press. Archived from the original on 2 November 2012. Retrieved 13 January 2011.
- 1 2 Harris, Gardiner (13 January 2011). "F.D.A. Plans New Limits on Prescription Painkillers". The New York Times. Archived from the original on 9 June 2012. Retrieved 13 January 2011.
- ↑ "Liquid paracetamol for children: revised UK dosing instructions introduced" (PDF). Medicines and Healthcare products Regulatory Agency (MHRA). 14 November 2011. Archived from the original (PDF) on 28 October 2019. Retrieved 27 October 2019.
- Lay summary in: http://www.mhra.gov.uk/safety-public-assessment-reports/CON221602.
{{cite web}}: Missing or empty|title=(help)
- Lay summary in: http://www.mhra.gov.uk/safety-public-assessment-reports/CON221602.
- ↑ Scialli AR, Ang R, Breitmeyer J, Royal MA (December 2010). "A review of the literature on the effects of acetaminophen on pregnancy outcome". Reprod. Toxicol. 30 (4): 495–507. doi:10.1016/j.reprotox.2010.07.007. PMID 20659550.
- ↑ Rudolph AM (23 February 1981). "Effects of aspirin and acetaminophen in pregnancy and in the newborn". Archives of Internal Medicine. 141 (3): 358–63. doi:10.1001/archinte.141.3.358. PMID 7469626.
- ↑ Eyers S, Weatherall M, Jefferies S, Beasley R (April 2011). "Paracetamol in pregnancy and the risk of wheezing in offspring: a systematic review and meta-analysis". Clinical and Experimental Allergy. 41 (4): 482–9. doi:10.1111/j.1365-2222.2010.03691.x. PMID 21338428.
- ↑ Blaser JA, Allan GM (July 2014). "Acetaminophen in pregnancy and future risk of ADHD in offspring". Canadian Family Physician. 60 (7): 642. PMC 4096264. PMID 25022638.
- ↑ de Fays L, Van Malderen K, De Smet K, Sawchik J, Verlinden V, Hamdani J, Dogné JM, Dan B (August 2015). "Use of paracetamol during pregnancy and child neurological development". Developmental Medicine & Child Neurology. 57 (8): 718–24. doi:10.1111/dmcn.12745. PMID 25851072.
- ↑ Choueiri TK, Je Y, Cho E (2014). "Analgesic use and the risk of kidney cancer: a meta-analysis of epidemiologic studies". International Journal of Cancer. 134 (2): 384–396. doi:10.1002/ijc.28093. PMC 3815746. PMID 23400756.
- 1 2 3 4 Fortuny J, Kogevinas M, Garcia-Closas M, Real FX, Tardón A, Garcia-Closas R, Serra C, Carrato A, Lloreta J, Rothman N, Villanueva C, Dosemeci M, Malats N, Silverman D (2006). "Use of Analgesics and Nonsteroidal Anti-inflammatory Drugs, Genetic Predisposition, and Bladder Cancer Risk in Spain". Cancer Epidemiology, Biomarkers & Prevention. 15 (9): 1696–1702. doi:10.1158/1055-9965.EPI-06-0038. PMID 16985032.
- ↑ Prescott LF (October 1980). "Kinetics and metabolism of paracetamol and phenacetin". British Journal of Clinical Pharmacology. 10 Suppl 2: 291S–298S. doi:10.1111/j.1365-2125.1980.tb01812.x. PMC 1430174. PMID 7002186.
- 1 2 Graham GG, Davies MJ, Day RO, Mohamudally A, Scott KF (June 2013). "The modern pharmacology of paracetamol: Therapeutic actions, mechanism of action, metabolism, toxicity, and recent pharmacological findings". Inflammopharmacology. 21 (3): 201–232. doi:10.1007/s10787-013-0172-x. PMID 23719833.
- 1 2 Marx, John; Walls, Ron; Hockberger, Robert (2013). Rosen's Emergency Medicine - Concepts and Clinical Practice. Elsevier Health Sciences. ISBN 9781455749874.
- 1 2 3 Ronald F., Borne (1995). "Nonsteroidal anti-inflammatory drugs". In Foye, William O.; Lemke, Thomas L.; Williams, David A (eds.). Principles of Medicinal Chemistry (Fourth ed.). Williams & Wilkins. pp. 544–545.
- 1 2 Brayfield, A., ed. (15 January 2014). "Paracetamol". Martindale: The Complete Drug Reference. London, UK: Pharmaceutical Press. Archived from the original on 28 August 2021. Retrieved 10 May 2014.
- ↑ Bales JR, Nicholson JK, Sadler PJ (1985). "Two-dimensional proton nuclear magnetic resonance "maps" of acetaminophen metabolites in human urine". Clinical Chemistry. 31 (5): 757–762. doi:10.1093/clinchem/31.5.757. PMID 3987005. Archived from the original on 21 November 2008. Retrieved 6 February 2009.
- 1 2 3 4 5 6 Bertolini A, Ferrari A, Ottani A, Guerzoni S, Tacchi R, Leone S (2006). "Paracetamol: New vistas of an old drug". CNS Drug Reviews. 12 (3–4): 250–75. doi:10.1111/j.1527-3458.2006.00250.x. PMC 6506194. PMID 17227290.
- ↑ Altinoz MA, Korkmaz R (2004). "NF-kappaB, macrophage migration inhibitory factor and cyclooxygenase-inhibitions as likely mechanisms behind the acetaminophen- and NSAID-prevention of the ovarian cancer". Neoplasma. 51 (4): 239–247. PMID 15254653.
- ↑ Bronwen, Byrant; Katleen, Knights; Evelyn, Salerno (2007). Pharmacology for health professionals. Elsevier. p. 270. ISBN 9780729537872.
- ↑ Ellis, Frank (2002). Paracetamol: a curriculum resource. Cambridge: Royal Society of Chemistry. ISBN 978-0-85404-375-0.
- ↑ Travis, Anthony S. (2007). "Manufacture and uses of the anilines: A vast array of processes and products". In Zvi Rappoport (ed.). The chemistry of Anilines Part 1. Wiley. p. 764. ISBN 978-0-470-87171-3.
- 1 2 Friderichs, Elmar; Christoph, Thomas; Buschmann, Helmut. "Analgesics and Antipyretics". Ullmann's Encyclopedia of Industrial Chemistry. Weinheim: Wiley-VCH. doi:10.1002/14356007.a02_269.pub2.
- ↑ US patent 4524217, Davenport, Kenneth G. & Hilton, Charles B., "Process for producing N-acyl-hydroxy aromatic amines", published 18 June 1985, assigned to Celanese Corporation
- ↑ Joncour R, Duguet N, Métay E, Ferreirab A, Lemaire M (2014). "Amidation of phenol derivatives: a direct synthesis of paracetamol (acetaminophen) from Hydroquinone". Green Chem. 16 (6): 2997–3002. doi:10.1039/C4GC00166D.
- ↑ Joncour, Roxan; Duguet, Nicolas; Métay, Estelle; Ferreira, Amadéo; Lemaire, Marc. "Supplementary Information Amidation of phenol derivatives: a direct synthesis of paracetamol (acetaminophen) from hydroquinone" (PDF). Archived (PDF) from the original on 24 September 2015.
- ↑ Henney K, Dudley B (1939). Handbook of Photography. Whittlesey House. p. 324.
- ↑ Novotny PE, Elser RC (1984). "Indophenol method for acetaminophen in serum examined" (PDF). Clin. Chem. 30 (6): 884–6. PMID 6723045.
- ↑ Cahn A, Hepp P (1886). "Das Antifebrin, ein neues Fiebermittel" [Antifebrin, a new antipyretic]. Centralblatt für klinische Medizin (in German). 7: 561–564. Archived from the original on 1 September 2020. Retrieved 5 August 2020.
{{cite journal}}: CS1 maint: unrecognized language (link) - ↑ Morse, H.N. (1878). "Ueber eine neue Darstellungsmethode der Acetylamidophenole" [On a new method of preparing acetylamidophenol]. Berichte der deutschen chemischen Gesellschaft (in German). 11 (1): 232–233. doi:10.1002/cber.18780110151. Archived from the original on 6 November 2018.
{{cite journal}}: CS1 maint: unrecognized language (link) - 1 2 3 4 5 Silverman, Milton; Lydecker, Mia; Lee, Philip Randolph (1992). Bad Medicine: The Prescription Drug Industry in the Third World. Stanford University Press. pp. 88–90. ISBN 978-0804716697.
- ↑ von Mering, J. (1893). "Beitrage zur Kenntniss der Antipyretica". Ther Monatsch. 7: 577–587.
- 1 2 3 4 Sneader, Walter (2005). Drug Discovery: A History. Hoboken, NJ: Wiley. p. 439. ISBN 978-0471899808. Archived from the original on 18 August 2016.
- ↑ Toussaint K, Yang XC, Zielinski MA, Reigle KL, Sacavage SD, Nagar S, Raffa RB (December 2010). "What do we (not) know about how paracetamol (acetaminophen) works?". Journal of Clinical Pharmacy and Therapeutics. 35 (6): 617–638. doi:10.1111/j.1365-2710.2009.01143.x. PMID 21054454.
- ↑ Bergman K, Müller L, Teigen SW (1996). "The genotoxicity and carcinogenicity of paracetamol: a regulatory (re)view". Mutat Res. 349 (2): 263–88. doi:10.1016/0027-5107(95)00185-9. PMID 8600357.
- ↑ Lester D, Greenberg LA, Carroll RP (1947). "The metabolic fate of acetanilid and other aniline derivatives: II. Major metabolites of acetanilid appearing in the blood". J. Pharmacol. Exp. Ther. 90 (1): 68–75. PMID 20241897. Archived from the original on 2 December 2008.
- ↑ Brodie BB, Axelrod J (1948). "The estimation of acetanilide and its metabolic products, aniline, N-acetyl p-aminophenol and p-aminophenol (free and total conjugated) in biological fluids and tissues". J. Pharmacol. Exp. Ther. 94 (1): 22–28. PMID 18885610.
- ↑ Brodie BB, Axelrod J (1948). "The fate of acetanilide in man" (PDF). J. Pharmacol. Exp. Ther. 94 (1): 29–38. PMID 18885611. Archived (PDF) from the original on 7 September 2008.
- ↑ Flinn FB, Brodie BB (1948). "The effect on the pain threshold of N-acetyl p-aminophenol, a product derived in the body from acetanilide". J. Pharmacol. Exp. Ther. 94 (1): 76–77. PMID 18885618.
- ↑ Brodie BB, Axelrod J (1949). "The fate of acetophenetidin (phenacetin) in man and methods for the estimation of acetophenitidin and its metabolites in biological material". J Pharmacol Exp Ther. 94 (1): 58–67.
- ↑ Landau, Ralph; Achilladelis, Basil; Scriabine, Alexander (1999). Pharmaceutical Innovation: Revolutionizing Human Health. Chemical Heritage Foundation. pp. 248–249. ISBN 978-0-941901-21-5. Archived from the original on 17 August 2016.
- ↑ Rapoport, Alan (15 December 1991). Headache Relief. Touchstone. p. 97. ISBN 978-0-671-74803-6. Archived from the original on 17 August 2016.
- ↑ "SEC Info - Eastman Kodak Co - '8-K' for 6/30/94". Archived from the original on 4 March 2016. Retrieved 3 March 2016.
- ↑ "Our Story". McNEIL-PPC, Inc. Archived from the original on 8 March 2014. Retrieved 8 March 2014.
- ↑ "Medication and Drugs". MedicineNet. 1996–2010. Archived from the original on 22 April 2010. Retrieved 22 April 2010.
- ↑ Thakkar KB, Billa G (September 2013). "The concept of: Generic drugs and patented drugs vs. brand name drugs and non-proprietary (generic) name drugs". Front Pharmacol. 4: 113. doi:10.3389/fphar.2013.00113. PMC 3770914. PMID 24062686.
- 1 2 Macintyre, Pamela; Rowbotham, David; Walker, Suellen (26 September 2008). Clinical Pain Management Second Edition: Acute Pain. CRC Press. p. 85. ISBN 978-0-340-94009-9. Archived from the original on 17 August 2016.
- ↑ "International Non-Proprietary Name for Pharmaceutical Preparations (Recommended List #4)" (PDF). WHO Chronicle. 16 (3): 101–111. March 1962. Archived (PDF) from the original on 18 May 2016. Retrieved 5 August 2020.
- ↑ "TGA Approved Terminology for Medicines, Section 1 – Chemical Substances" (PDF). Therapeutic Goods Administration, Department of Health and Ageing, Australian Government. July 1999: 97. Archived (PDF) from the original on 11 February 2014.
{{cite journal}}: Cite journal requires|journal=(help) - ↑ "Les prix en Europe - La boîte paracétamol 10 comprimés (générique)" (in français). Linternaute.com. Archived from the original on 25 August 2018. Retrieved 10 October 2018.
- ↑ "Acetaminophen" in Physicians' Desk Reference, 63rd ed. Montvale, NJ: Thomson PDR. 2009. pp. 1915–1916. ISBN 978-1563637032
- ↑ "Acetaminophen Overdose and Liver Injury — Background and Options for Reducing Injury" (PDF). U.S. Food and Drug Administration (FDA). 22 May 2009. Archived (PDF) from the original on 19 October 2014.
 This article incorporates text from this source, which is in the public domain. Background Package for 29–30 June 2009 Meeting of the Drug Safety and Risk Management Committee, Anesthetic and Life Support Drugs Advisory Committee and Nonprescription Drugs Advisory Committee
This article incorporates text from this source, which is in the public domain. Background Package for 29–30 June 2009 Meeting of the Drug Safety and Risk Management Committee, Anesthetic and Life Support Drugs Advisory Committee and Nonprescription Drugs Advisory Committee - ↑ Nam, Stephanie. "IV, PO, and PR Acetaminophen: A Quick Comparison". Pharmacy Times. Archived from the original on 24 October 2019. Retrieved 24 October 2019.
- ↑ "Ofirmev Prices and Ofirmev Coupons - GoodRx". GoodRx. Archived from the original on 30 November 2021. Retrieved 30 November 2021.
- ↑ "Acetaminophen and Codeine (Professional Patient Advice)". Drugs.com. 29 June 2019. Archived from the original on 20 May 2020. Retrieved 25 February 2020.
- ↑ "Acetaminophen, Caffeine, and Dihydrocodeine (Professional Patient Advice)". Drugs.com. 2 October 2019. Archived from the original on 19 May 2020. Retrieved 25 February 2020.
- ↑ "Oxycodone and Acetaminophen (Professional Patient Advice)". Drugs.com. 11 November 2019. Archived from the original on 20 May 2020. Retrieved 25 February 2020.
- ↑ "Hydrocodone and Acetaminophen (Professional Patient Advice)". Drugs.com. 2 January 2020. Archived from the original on 21 May 2020. Retrieved 25 February 2020.
- ↑ "Propoxyphene and Acetaminophen Tablets". Drugs.com. 21 June 2019. Archived from the original on 20 May 2020. Retrieved 25 February 2020.
- ↑ "APOHealth Paracetamol Plus Codeine & Calmative". Drugs.com. 3 February 2020. Archived from the original on 25 February 2020. Retrieved 25 February 2020.
- 1 2 Atkinson HC, Stanescu I, Anderson BJ (2014). "Increased Phenylephrine Plasma Levels with Administration of Acetaminophen". New England Journal of Medicine. 370 (12): 1171–1172. doi:10.1056/NEJMc1313942. PMID 24645960.
- ↑ "Ascorbic acid/Phenylephrine/Paracetamol". NHS Choices. National Health Service. Archived from the original on 26 March 2014. Retrieved 25 March 2014.
- ↑ "Phenylephrine/Caffeine/Paracetamol dual relief". NHS Choices. National Health Service. Archived from the original on 26 March 2014. Retrieved 25 March 2014.
- ↑ "Beechams Decongestant Plus With Paracetamol". NHS Choices. National Health Service. Archived from the original on 26 March 2014. Retrieved 25 March 2014.
- ↑ Senyuva H, Ozden T (2002). "Simultaneous High-Performance Liquid Chromatographic Determination of Paracetamol, Phenylephrine HCl, and Chlorpheniramine Maleate in Pharmaceutical Dosage Forms". Journal of Chromatographic Science. 40 (2): 97–100. doi:10.1093/chromsci/40.2.97. PMID 11881712.
- ↑ Janin A, Monnet J (2014). "Bioavailability of paracetamol, phenylephrine hydrochloride and guaifenesin in a fixed-combination syrup versus an oral reference product". Journal of International Medical Research. 42 (2): 347–359. doi:10.1177/0300060513503762. PMID 24553480.
- ↑ "Paracetamol – phenylephrine hydrochloride – guaifenesin". NPS MedicineWise. National Prescribing Service (Australia). Archived from the original on 26 March 2014. Retrieved 25 March 2014.
- ↑ "Phenylephrine/Guaifenesin/Paracetamol". NHS Choices. National Health Service. Archived from the original on 12 September 2013. Retrieved 25 March 2014.
- ↑ "Treating a Restless Legs Sydnrome (RLS)". Consumer Reports. 2011. Archived from the original on 18 December 2015.
- ↑ "Use Only as Directed". This American Life. Episode 505. Chicago. 20 September 2013. Public Radio International. WBEZ. Archived from the original on 27 September 2013. Retrieved 24 September 2013.
{{cite episode}}: Unknown parameter|serieslink=ignored (help) - ↑ Gerth, Jeff; T. Christian Miller (20 September 2013). "Use Only as Directed". ProPublica. Archived from the original on 24 September 2013. Retrieved 24 September 2013.
- ↑ Miller, T. Christian; Gerth, Jeff (20 September 2013). "Dose of Confusion". ProPublica. Archived from the original on 24 September 2013. Retrieved 24 September 2013.
- ↑ "Recommendations for FDA Interventions to Decrease the Occurrence of Acetaminophen Hepatotoxicity" (PDF). Archived (PDF) from the original on 24 September 2015.
 This article incorporates text from this source, which is in the public domain.
This article incorporates text from this source, which is in the public domain. - ↑ Allen AL (2003). "The diagnosis of acetaminophen toxicosis in a cat". Can Vet J. 44 (6): 509–10. PMC 340185. PMID 12839249.
- 1 2 3 Richardson, JA (2000). "Management of acetaminophen and ibuprofen toxicoses in dogs and cats" (PDF). J. Vet. Emerg. Crit. Care. 10 (4): 285–91. doi:10.1111/j.1476-4431.2000.tb00013.x. Archived from the original (PDF) on 1 April 2010.
- 1 2 Maddison, Jill E.; Stephen W. Page; Church, David (2002). Small Animal Clinical Pharmacology. Elsevier Health Sciences. pp. 260–1. ISBN 978-0702025730.
- 1 2 3 "Pardale-V Oral Tablets". NOAH Compendium of Data Sheets for Animal Medicines. The National Office of Animal Health (NOAH). 11 November 2010. Archived from the original on 22 November 2008. Retrieved 20 January 2011.
- ↑ Villar D, Buck WB, Gonzalez JM (1998). "Ibuprofen, aspirin and acetaminophen toxicosis and treatment in dogs and cats". Vet Hum Toxicol. 40 (3): 156–62. PMID 9610496.
- ↑ Gwaltney-Brant S, Meadows I (March 2006). "The 10 Most Common Toxicoses in Dogs". Veterinary Medicine: 142–8. Archived from the original on 10 July 2011. Retrieved 28 October 2019.
- ↑ Dunayer, E (2004). "Ibuprofen toxicosis in dogs, cats, and ferrets". Veterinary Medicine: 580–6. Archived from the original on 10 July 2011.
- ↑ Johnston J, Savarie P, Primus T, Eisemann J, Hurley J, Kohler D (2002). "Risk assessment of an acetaminophen baiting program for chemical control of brown tree snakes on Guam: evaluation of baits, snake residues, and potential primary and secondary hazards". Environ Sci Technol. 36 (17): 3827–33. Bibcode:2002EnST...36.3827J. doi:10.1021/es015873n. PMID 12322757.
- ↑ Lendon, Brad (7 September 2010). "Tylenol-loaded mice dropped from air to control snakes". CNN. Archived from the original on 9 September 2010. Retrieved 7 September 2010.
- ↑ Richards, Sabrina (1 May 2012). "It's Raining Mice". The Scientist. Archived from the original on 15 May 2012.
External links
- "Acetaminophen". Drug Information Portal. U.S. National Library of Medicine. Archived from the original on 6 August 2020. Retrieved 5 August 2020.
- "Acetaminophen: Avoiding Liver Injury". U.S. Food and Drug Administration (FDA). 24 June 2009. Archived from the original on 6 August 2020. Retrieved 5 August 2020.
| Identifiers: |
|---|