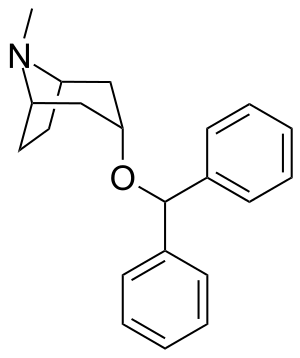
Benzatropine
 | |
 | |
| Names | |
|---|---|
| Trade names | Cogentin, others |
| Other names | Benztropine, benztropine (BAN UK), benztropine (USAN US) |
IUPAC name
| |
| Clinical data | |
| Drug class | Anticholinergic[1] |
| Main uses | Dystonia, parkinsonism[1] |
| Side effects | Dry mouth, blurry vision, nausea, constipation[1] |
| WHO AWaRe | UnlinkedWikibase error: ⧼unlinkedwikibase-error-statements-entity-not-set⧽ |
| Pregnancy category |
|
| Routes of use | By mouth, IM, IV |
| Defined daily dose | 2 mg[2] |
| External links | |
| AHFS/Drugs.com | Monograph |
| Legal | |
| License data |
|
| Legal status |
|
| Pharmacokinetics | |
| Metabolism | Liver |
| Elimination half-life | 12-24 hours |
| Excretion | Urine |
| Chemical and physical data | |
| Formula | C21H25NO |
| Molar mass | 307.437 g·mol−1 |
| 3D model (JSmol) | |
SMILES
| |
InChI
| |
Benzatropine, sold under the brand name Cogentin among others, is a medication used to treat a movement disorder known as dystonia which results from antipsychotics and parkinsonism.[1] It is not useful for tardive dyskinesia.[1] It is taken by mouth or by injection into a vein or muscle.[1] Benefits are seen within two hours and last for up to ten hours.[3][4]
Common side effects include dry mouth, blurry vision, nausea, and constipation.[1] Serious side effect may include urinary retention, hallucinations, hyperthermia, and poor coordination.[1] It is unclear if use during pregnancy or breastfeeding is safe.[5] Benzatropine is an anticholinergic which works by blocking the activity of the muscarinic acetylcholine receptor.[1]
Benzatropine was approved for medical use in the United States in 1954.[1] It is available as a generic medication.[1] In the United States the wholesale cost is about US$6 per month.[6] In 2017, it was the 226th most commonly prescribed medication in the United States, with more than two million prescriptions.[7][8]
Medical uses
Benzatropine is used to reduce extrapyramidal side effects of antipsychotic treatment. Benzatropine is also a second-line drug for the treatment of Parkinson's disease. It improves tremor, and may alleviate rigidity and bradykinesia.[9] Benzatropine is also sometimes used for the treatment of dystonia, a rare disorder that causes abnormal muscle contraction, resulting in twisting postures of limbs, trunk, or face.
Dosage
The defined daily dose is 2 mg by mouth or by injection.[2]
Side effects
These are principally anticholinergic:
- Dry mouth
- Blurred vision
- Cognitive changes
- Drowsiness
- Constipation
- Urinary retention
- Fast heartrate
- Loss of appetite
- Delirium and hallucinations (in overdose)
While some studies suggest that use of anticholinergics increases the risk of tardive dyskinesia (a long-term side effect of antipsychotics),[10][11] other studies have found no association between anticholinergic exposure and risk of developing tardive dyskinesia,[12] although symptoms may be worsened.[13]
Drugs that decrease cholinergic transmission may impair storage of new information into long-term memory. Anticholinergic agents can also impair time perception.[14]
Pharmacology
Benzatropine is a centrally acting anticholinergic/antihistamine agent. It is a selective M1 muscarinic acetylcholine receptor antagonist. Benzatropine partially blocks cholinergic activity in the basal ganglia and has also been shown to increase the availability of dopamine by blocking its reuptake and storage in central sites, and as a result, increasing dopaminergic activity. Animal studies have indicated that anticholinergic activity of benzatropine is approximately one-half that of atropine, while its antihistamine activity approaches that of mepyramine. Its anticholinergic effects have been established as therapeutically significant in the management of Parkinsonism. Benzatropine antagonizes the effect of acetylcholine, decreasing the imbalance between the neurotransmitters acetylcholine and dopamine, which may improve the symptoms of early Parkinson's disease.[15]
Benzatropine analogues are atypical dopamine reuptake inhibitors,[16] which might make them useful for people with akathisia secondary to antipsychotic therapy.[17]
Benzatropine also acts as a functional inhibitor of acid sphingomyelinase (FIASMA).[18]
Benzatropine has been also identified, by a high throughput screening approach, as a potent differentiating agent for oligodendrocytes, possibly working through M1 and M3 muscarinic receptors. In preclinical models for multiple sclerosis, benzatropine decreased clinical symptoms and enhanced re-myelination.[19]
Society and culture
Cost
In the United States the wholesale cost is about US$6 per month.[6] In 2017, it was the 226th most commonly prescribed medication in the United States, with more than two million prescriptions.[7][8]
.svg.png.webp) Costs (US)
Costs (US).svg.png.webp) Prescriptions (US)
Prescriptions (US)
Other animals
In veterinary medicine, benzatropine is used to treat priapism in stallions.[20]
References
- 1 2 3 4 5 6 7 8 9 10 11 "Benztropine Mesylate Monograph for Professionals". Drugs.com. American Society of Health-System Pharmacists. Archived from the original on 6 June 2019. Retrieved 9 April 2019.
- 1 2 "WHOCC - ATC/DDD Index". www.whocc.no. Archived from the original on 7 August 2020. Retrieved 7 September 2020.
- ↑ Pagliaro, Louis A.; Pagliaro, Ann M. (1999). PNDR, Psychologists' Neuropsychotropic Drug Reference. Psychology Press. p. 47. ISBN 9780876309568. Archived from the original on 2020-07-26. Retrieved 2019-04-09.
- ↑ Aschenbrenner, Diane S.; Venable, Samantha J. (2009). Drug Therapy in Nursing. Lippincott Williams & Wilkins. p. 197. ISBN 9780781765879. Archived from the original on 2020-07-26. Retrieved 2019-04-09.
- ↑ "Benztropine (Cogentin) Use During Pregnancy". Drugs.com. Archived from the original on 6 June 2019. Retrieved 9 April 2019.
- 1 2 "NADAC as of 2019-02-27". Centers for Medicare and Medicaid Services. Archived from the original on 2019-03-06. Retrieved 3 March 2019.
- 1 2 "The Top 300 of 2020". ClinCalc. Archived from the original on 12 February 2021. Retrieved 11 April 2020.
- 1 2 "Benztropine Mesylate - Drug Usage Statistics". ClinCalc. Archived from the original on 3 July 2020. Retrieved 11 April 2020.
- ↑ DiMascio, A.; Bernardo, D. L.; Greenblatt, D. J.; Marder, J. E. (1976). "A controlled trial of amantadine in drug-induced extrapyramidal disorders". Archives of General Psychiatry. 33 (5): 599–602. doi:10.1001/archpsyc.1976.01770050055008. ISSN 0003-990X. PMID 5066.
- ↑ Kane JM, Smith JM (1982). "Tardive dyskinesia: Prevalence and risk factors, 1959 to 1979". Archives of General Psychiatry. 39 (4): 473–81. doi:10.1001/archpsyc.1982.04290040069010. PMID 6121548.
- ↑ Wszola BA, Newell KM, Sprague RL (2001). "Risk factors for tardive dyskinesia in a large population of youths and adults". Experimental and Clinical Psychopharmacology. 9 (3): 285–96. doi:10.1037/1064-1297.9.3.285. PMID 11534539.
- ↑ van Harten PN, Hoek HW, Matroos GE, Koeter M, Kahn RS (1998). "Intermittent neuroleptic treatment and risk for tardive dyskinesia: Curaçao Extrapyramidal Syndromes Study III". The American Journal of Psychiatry. 155 (4): 565–7. doi:10.1176/ajp.155.4.565. PMID 9546009.
- ↑ Yassa R (1988). "Tardive dyskinesia and anticholinergic drugs. A critical review of the literature". L'Encéphale. 14 (Spec No): 233–9. PMID 3063514.
- ↑ Gelenberg AJ, Van Putten T, Lavori PW, Wojcik JD, Falk WE, Marder S, Galvin-Nadeau M, Spring B, Mohs RC, Brotman AW (1989). "Anticholinergic effects on memory: benztropine versus amantadine". Clinical Psychopharmacology. 9 (3): 180–5. doi:10.1097/00004714-198906000-00004. PMID 2661606.
- ↑ MIMS Australia Pty Ltd. MIMS.
- ↑ Hiranita T, Kohut SJ, Soto PL, Tanda G, Kopajtic TA, Katz JL (2014). "Preclinical efficacy of N-substituted benztropine analogs as antagonists of methamphetamine self-administration in rats". J Pharmacol Exp Ther. 348 (1): 174–91. doi:10.1124/jpet.113.208264. PMC 3868882. PMID 24194527.
- ↑ Adler LA, Peselow E, Rosenthal M, Angrist B (1993). "A controlled comparison of the effects of propranolol, benztropine, and placebo on akathisia: an interim analysis". Psychopharmacol Bull. 29: 283–6. PMID 8290678.
- ↑ Kornhuber J, Muehlbacher M, Trapp S, Pechmann S, Friedl A, Reichel M, Mühle C, Terfloth L, Groemer T, Spitzer G, Liedl K, Gulbins E, Tripal P (2011). "Identification of novel functional inhibitors of acid sphingomyelinase". PLoS ONE. 6 (8): e23852. doi:10.1371/journal.pone.0023852. PMC 3166082. PMID 21909365.
- ↑ Deshmukh VA, Tardif V, Lyssiotis CA, Green CC, Kerman B, Kim HJ, Padmanabhan K, Swoboda JG, Ahmad I, Kondo T, Gage FH, Theofilopoulos AN, Lawson BR, Schultz PG, Lairson LL (2013). "A regenerative approach to the treatment of multiple sclerosis". Nature. 502 (7471): 327–332. doi:10.1038/nature12647. PMC 4431622. PMID 24107995.
- ↑ Wilson, DV; Nickels, FA; Williams, MA (1 Nov 1991). "Pharmacologic treatment of priapism in two horses". Journal of the American Veterinary Medical Association: 1183–4.
External links
| External sites: |
|
|---|---|
| Identifiers: |