Budesonide
 | |
 | |
| Names | |
|---|---|
| Trade names | Pulmicort, Rhinocort, Entocort, others |
IUPAC name
| |
| Clinical data | |
| Drug class | Corticosteroid[1] |
| Main uses | Asthma, COPD, nasal polyps, inflammatory bowel disease[2][1][3] |
| Side effects | Inhaled: Respiratory infections, cough, headaches[1] Pills: Feeling tired, vomiting, joint pains[1] |
| WHO AWaRe | UnlinkedWikibase error: ⧼unlinkedwikibase-error-statements-entity-not-set⧽ |
| Pregnancy category |
|
| Routes of use | By mouth, nasal, tracheal, rectal, inhalation |
| Defined daily dose | 0.8 mg (inhalation aerosol or powder) 1.5 mg (inhalation solution)[4] 0.2 mg (nasal)[5] 9 mg (by mouth)[6] |
| External links | |
| AHFS/Drugs.com | Inhaled: Monograph Nose: Monograph |
| MedlinePlus | a608007 |
| Legal | |
| License data |
|
| Legal status | |
| Pharmacokinetics | |
| Bioavailability | 10-20% (first pass effect) |
| Protein binding | 85-90% |
| Metabolism | Hepatic CYP3A4 |
| Elimination half-life | 2.0-3.6 hours |
| Excretion | Urine, feces |
| Chemical and physical data | |
| Formula | C25H34O6 |
| Molar mass | 430.541 g·mol−1 |
| 3D model (JSmol) | |
SMILES
| |
InChI
| |
Budesonide (BUD), sold under the brand name Pulmicort among others, is a medication of the corticosteroid type.[1] It is available as an inhaler, pill, nasal spray, and rectal forms.[1][2] The inhaled form is used in the long-term management of asthma and chronic obstructive pulmonary disease(COPD) and in COVID-19.[1][7][8][9] The nasal spray is used for allergic rhinitis and nasal polyps.[2][10] The pills in a delayed release form and rectal forms may be used for inflammatory bowel disease including Crohn's disease, ulcerative colitis and microscopic colitis.[3][11][12]
Common side effects with the inhaled form include respiratory infections, cough, and headaches.[1] Common side effects with the pills include feeling tired, vomiting, and joint pains.[1] Serious side effects include an increased risk of infection, loss of bone strength, and cataracts.[1] Long-term use of the pill form may cause adrenal insufficiency.[1] Stopping the pills suddenly following long-term use may therefore be dangerous.[1] The inhaled form is generally safe in pregnancy.[1] Budesonide is mainly acting as a glucocorticoid.[1]
Budesonide was initially patented in 1973.[13] Commercial use as an asthma medication began in 1981.[14] It is on the World Health Organization's List of Essential Medicines.[15] Some forms are available as a generic medication.[16] The wholesale price in the developing world for an inhaler containing 200 doses is about US$5 to US$7 as of 2014.[17] As of 2015, the cost for a typical month of the inhaler medication in the United States is US$100 to US$200.[16] In 2019, generic budesonide was listed as involved in Teva's price fixing scheme in the USA.[18] In 2017, it was the 190th most commonly prescribed medication in the United States, with more than three million prescriptions.[19][20]
Medical uses
Asthma
Budesonide is given by metered-dose inhaler or nebulizer for maintenance and prophylactic treatment of asthma including patients who require oral corticosteroids and those who may benefit from a systemic dose reduction.[21]
Inflammatory bowel disease
Formulations of delayed-release budesonide are an effective treatment for mild-to-moderately active Crohn's disease involving the ileum and/or ascending colon.[22] A Cochrane review found evidence for up to three months (but not longer) of maintenance of remission in Crohn's disease.[23]
Budesonide assists in the induction of remission in people with active ulcerative colitis.[24]
Budesonide is highly effective and recommended as the drug of choice in microscopic colitis, for induction and maintenance of remission, and for both the lymphocytic colitis and collagenous colitis forms.[25]
Allergic rhinitis
Budesonide in the form of nasal sprays is a treatment for allergic rhinitis.[26]
Eosinophilic esophagitis
Topical budesonide has considerable effects in eosinophilic esophagitis.[27] For this use, it is formulated as a tablet that disperses in the mouth.[28]
COVID-19
There is tentative evidence that budesonide may be useful in those with mild COVID-19, but at high risk of getting worse.[29][9] Evidence as of October 2021; however, is insufficient to recommend either for or against its use.[29]
Dosage
The defined daily dose is 0.8 mg (inhalation aerosol) or 800 ucg (inhalation powder) or 1.5 mg (inhalation solution) for asthma[4] and 0.2 mg (nasal) [5] and 9 mg (by mouth).[6] The inhaled powder has the benefit of releasing less green house gases.[30]
For COVID a dose of 800 ug twice per day for 2 weeks may be used.[31]
Side effects
Budesonide may cause:[32]
- Nose irritation or burning
- Bleeding or sores in the nose
- Lightheadedness
- Upset stomach
- Cough
- Hoarseness
- Dry mouth
- Rash
- Sore throat
- Bad taste in mouth
- Change in mucus
- Blurred vision [33]
In addition, the following symptoms should be reported immediately:
- Difficulty breathing or swelling of the face
- White patches in the throat, mouth, or nose
- Irregular menstrual periods
- Severe acne
- On rare occasions, behavioral changes (mostly affecting children)[32]
Contraindications
Budesonide is contraindicated as a primary treatment of status asthmaticus or other acute episode of asthma where intensive measures are required.[34] It is also contraindicated for patients who have hypersensitivity to budesonide.[35]
Interactions
Those taking tablets or capsules orally should avoid grapefruit juice and echinacea.
- Grapefruit juice may double bioavailability of oral budesonide.
- Echinacea diminishes bioavailability.
Also, high-fat meals delay absorption but do not impede absorption.
Pharmacology
Budesonide is an agonist of glucocorticoid receptors. Among its effects are:
- Controls the rate of protein synthesis.
- Depresses the migration of polymorphonuclear leukocytes and fibroblasts.
- Reverses capillary permeability and lysosomal stabilization at the cellular level to prevent or control inflammation.
- Has a potent glucocorticoid activity and weak mineralocorticoid activity.
Pharmacokinetics
- Onset of action: Nebulization: 2–8 days; Inhalation: 24 hours; Nasal: 10 hours
- Peak effect: Nebulization: 4–6 weeks; Inhalation: 1–2 weeks
- Distribution: 2.2-3.9 L/kg
- Protein binding: 85% to 90%
- Metabolism: Hepatic via CYP3A4 to two metabolites: 16 alpha-hydroxyprednisolone and 6 beta-hydroxybudesonide; minor activity
- Bioavailability: Limited by high first-pass effect; Capsule: 9% to 21%; Nebulization: 6%; Inhalation: 6% to 13%
- Half-life elimination: 2–3.6 hours
- Time to peak: Capsule: 0.5–10 hours (variable in Crohn's disease); Nebulization: 10–30 minutes; Inhalation: 1–2 hours; Tablet: 7.4-19.2 hours
- Excretion: urine (60%) and feces as metabolites.
Chemistry
Budesonide, also known as 11β,21-dihydroxy-16α,17α-(butylidenebis(oxy))pregna-1,4-diene-3,20-dione, is a synthetic pregnane steroid and non-halogenated cyclic ketal corticosteroid.[36][37] It is the C16α hydroxyl, C16α,17α cyclic ketal with butyraldehyde derivative of prednisolone (11β,17α,21-trihydroxypregna-1,4-diene-3,20-dione).[36][37]
Stereoisomerism
| Budesonide (2 stereoisomers) | |
|---|---|
 (22R)-configuration |
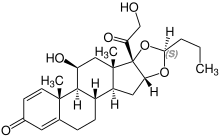 (22S)-configuration |
Society and culture
Cost
The wholesale price in the developing world for an inhaler containing 200 doses is about US$5 to US$7 as of 2014.[17] As of 2015, the cost for a typical month of the inhaler medication in the United States is US$100 to US$200.[16] In 2017, it was the 190th most commonly prescribed medication in the United States, with more than three million prescriptions.[19][20]
.svg.png.webp) Budesonide costs (US)
Budesonide costs (US).svg.png.webp) Budesonide prescriptions (US)
Budesonide prescriptions (US)
Brand names

Aeronide (TH); Aquacort (DE); B Cort (CO); Bronex (PH); Budair (MY); Budecort DP (MY); Budenofalk (DE, GB, HK, KP, PH, SG); Budeson (AR); Budeson Aqua (AR); BudeSpray (TH); Budiair (KP); Budicort Respules (IL); Budinide (KSA); Bunase (TH); Clebudan (CN); Cortiment (GB); Cycortide (HK); Denecort (PH); Duasma (TW); Eltair (MY); Entocort (AR, AT, BE, BR, CH, CZ, DK, FI, FR, GB, HK, IE, IL, IT, KP, NL, NO, PL, PT, SE, TR); Giona Easyhaler (MY, SG, TH); Inflammide (PE); Miflonid (CZ); Miflonide (BE, DE, IL, IT, NZ, PT); Neumocort (PY); Novopulmon (DE, FR); Pulmicon Susp for Nebulizer (KP); Pulmicort (AT, BE, BG, BR, CH, CL, CN, CO, CR, CZ, DE, DK, DO, EE, FI, FR, GB, GR, GT, HN, ID, IN, NI, NL, NO, PA, PK, PL, PT, RU, SE, SV, TR, TW, UY, VE, ZA); Pulmicort Nasal Turbohaler (CL, KE, MU, NG); Pulmicort Turbuhaler (KE, MU, NG); Rafton (FR); Rhinocort (AU); Rhinocort Aqua (HK); Rhinoside (GR); Symbicort (FR, US, ZA) Uceris (US)
See also
References
- 1 2 3 4 5 6 7 8 9 10 11 12 13 14 "Budesonide". The American Society of Health-System Pharmacists. Archived from the original on 28 November 2015. Retrieved 2 December 2015.
- 1 2 3 "Budesonide eent". The American Society of Health-System Pharmacists. Archived from the original on 8 December 2015. Retrieved 2 December 2015.
- 1 2 Silverman J, Otley A (2011). "Budesonide in the treatment of inflammatory bowel disease". Expert Rev Clin Immunol. 7 (4): 419–28. doi:10.1586/eci.11.34. PMID 21790284.
- 1 2 "WHOCC - ATC/DDD Index". www.whocc.no. Archived from the original on 20 September 2020. Retrieved 22 September 2020.
- 1 2 "WHOCC - ATC/DDD Index". www.whocc.no. Archived from the original on 20 September 2020. Retrieved 22 September 2020.
- 1 2 "WHOCC - ATC/DDD Index". www.whocc.no. Archived from the original on 21 October 2020. Retrieved 22 September 2020.
- ↑ De Coster, DA; Jones, M (2014). "Tailoring of corticosteroids in COPD management". Current Respiratory Care Reports. 3 (3): 121–132. doi:10.1007/s13665-014-0084-2. PMC 4113685. PMID 25089228.
- ↑ Christophi, GP; Rengarajan, A; Ciorba, MA (2016). "Rectal budesonide and mesalamine formulations in active ulcerative proctosigmoiditis: efficacy, tolerance, and treatment approach". Clinical and Experimental Gastroenterology. 9: 125–30. doi:10.2147/CEG.S80237. PMC 4876845. PMID 27274301.
- 1 2 Ton, Joey (13 December 2021). "#304 Budesonide Bests COVID-19". CFPCLearn. Archived from the original on 28 March 2023. Retrieved 14 June 2023.
- ↑ Rudmik, L; Schlosser, RJ; Smith, TL; Soler, ZM (July 2012). "Impact of topical nasal steroid therapy on symptoms of nasal polyposis: a meta-analysis". The Laryngoscope. 122 (7): 1431–7. doi:10.1002/lary.23259. PMID 22410935.
- ↑ Pardi DS, Tremaine WJ, Carrasco-Labra A (2016). "American Gastroenterological Association Institute Technical Review on the Medical Management of Microscopic Colitis" (PDF). Gastroenterology. 150 (1): 247–274.e11. doi:10.1053/j.gastro.2015.11.006. PMID 26584602. Archived from the original on 28 August 2021. Retrieved 5 September 2019.
- ↑ British national formulary : BNF 58 (58 ed.). British Medical Association. 2009. pp. 56–57. ISBN 9780857111562.
- ↑ Domeij, Bengt (2000). Pharmaceutical patents in Europe. The Hague: Kluwer Law International. p. 278. ISBN 9789041113481. Archived from the original on 8 December 2015.
- ↑ Hamley, Peter (2015). Small Molecule Medicinal Chemistry: Strategies and Technologies. John Wiley & Sons. p. 390. ISBN 9781118771693. Archived from the original on 8 December 2015.
- ↑ World Health Organization (2019). World Health Organization model list of essential medicines: 21st list 2019. Geneva: World Health Organization. hdl:10665/325771. WHO/MVP/EMP/IAU/2019.06. License: CC BY-NC-SA 3.0 IGO.
- 1 2 3 Hamilton, Richart (2015). Tarascon Pocket Pharmacopoeia 2015 Deluxe Lab-Coat Edition. Jones & Bartlett Learning. p. 451. ISBN 9781284057560.
- 1 2 "Budesonide". International Drug Price Indicator Guide. Archived from the original on 11 July 2020. Retrieved 5 December 2015.
- ↑ Murphy, Heather (11 May 2019). "Teva and Other Generic Drugmakers Inflated Prices Up to 1,000%, State Prosecutors Say". The New York Times. ISSN 0362-4331. Archived from the original on 30 May 2020. Retrieved 27 May 2020.
- 1 2 "The Top 300 of 2020". ClinCalc. Archived from the original on 12 February 2021. Retrieved 11 April 2020.
- 1 2 "Budesonide - Drug Usage Statistics". ClinCalc. Archived from the original on 29 April 2017. Retrieved 11 April 2020.
- ↑ Global Strategy for Asthma Management and Prevention, Global Initiative for Asthma (GINA) 2011. Available at http://www.ginasthma.org Archived 2013-10-14 at the Wayback Machine
- ↑ Lichtenstein GR, Hanauer SB, Sandborn WJ (2009). "Management of Crohn's Disease in Adults". Am J Gastroenterol. 104 (2): 465–83. doi:10.1038/ajg.2008.168. PMID 19174807.
- ↑ Kuenzig ME, Rezaie A, Seow CH, Otley AR, Steinhart AH, Griffiths AM, et al. (2014). "Budesonide for maintenance of remission in Crohn's disease". Cochrane Database Syst Rev. 8 (8): CD002913. doi:10.1002/14651858.CD002913.pub3. PMC 7133546. PMID 25141071.
- ↑ Habal FM, Huang VW (2012). "Review Article: A Decision-Making Algorithm For the Management of Pregnancy in the Inflammatory Bowel Disease Patient". Aliment Pharmacol Ther. 35 (5): 501–15. doi:10.1111/j.1365-2036.2011.04967.x. PMID 22221203.
- ↑ Pardi, Darrell (2016). "American Gastroenterological Association Institute Technical Review on the Medical Management of Microscopic Colitis". www.gastrojournal.org. Archived from the original on 4 February 2021.
- ↑ Stanaland, BE (April 2004). "Once-daily budesonide aqueous nasal spray for allergic rhinitis: a review". Clinical Therapeutics. 26 (4): 473–92. doi:10.1016/s0149-2918(04)90050-1. PMID 15189745.
- ↑ Rawla, P; Sunkara, T; Thandra, KC; Gaduputi, V (December 2018). "Efficacy and Safety of Budesonide in the Treatment of Eosinophilic Esophagitis: Updated Systematic Review and Meta-Analysis of Randomized and Non-Randomized Studies". Drugs in R&D. 18 (4): 259–269. doi:10.1007/s40268-018-0253-9. PMC 6277325. PMID 30387081.
- ↑ UK Drug Information
- 1 2 "Corticosteroids". COVID-19 Treatment Guidelines. Archived from the original on 11 December 2021. Retrieved 16 December 2021.
- ↑ CASCADES - Primary Care Toolkit_EN - Page 28. 2023. p. 28. Archived from the original on 2 July 2023. Retrieved 30 June 2023.
- ↑ "Treatments". www.bccdc.ca. Archived from the original on 9 October 2021. Retrieved 3 January 2022.
- 1 2 Budesonide - Nasal Aerosol Inhaler (Rhinocort) side effects, medical uses, and drug interactions Archived 6 November 2008 at the Wayback Machine
- ↑ "Budesonide: CMDh scientific conclusions and grounds for variation, amendments to the product information and timetable for the implementation - PSUSA/00000449/201604" (PDF). European Medicines Agency (EMA). 10 March 2017. Archived (PDF) from the original on 8 September 2017. Retrieved 19 May 2017.
- ↑ Todd GR, Acerini CL, Buck JJ, Murphy NP, Ross-Russell R, Warner JT, McCance DR (2002). "Acute Adrenal Crisis in Asthmatics Treated With High-Dose Fluticasone Propionate". Eur Respir J. 19 (6): 1207–9. doi:10.1183/09031936.02.00274402. PMID 12108877.
- ↑ Todd GR, Acerini CL, Ross-Russell R, Zahra S, Warner JT, McCance D (2002). "Survey of Adrenal Crisis Associated With Inhaled Corticosteroids in the United Kingdom". Arch Dis Child. 87 (6): 457–61. doi:10.1136/adc.87.6.457. PMC 1755820. PMID 12456538.
- 1 2 J. Elks (14 November 2014). The Dictionary of Drugs: Chemical Data: Chemical Data, Structures and Bibliographies. Springer. pp. 186, 1011. ISBN 978-1-4757-2085-3. Archived from the original on 8 September 2017.
- 1 2 Thomas L. Lemke; David A. Williams (2008). Foye's Principles of Medicinal Chemistry. Lippincott Williams & Wilkins. pp. 1253–. ISBN 978-0-7817-6879-5. Archived from the original on 8 September 2017.
External links
| External sites: |
|
|---|---|
| Identifiers: |
- "Budesonide Nasal Spray". MedlinePlus. Archived from the original on 30 April 2017. Retrieved 9 February 2020.
- "Budesonide Oral Inhalation". MedlinePlus. Archived from the original on 30 April 2017. Retrieved 9 February 2020.