Doxpicomine
 | |
| Clinical data | |
|---|---|
| Routes of administration | Oral |
| ATC code |
|
| Legal status | |
| Legal status |
|
| Identifiers | |
IUPAC name
| |
| CAS Number |
|
| PubChem CID | |
| ChemSpider | |
| UNII |
|
| KEGG | |
| Chemical and physical data | |
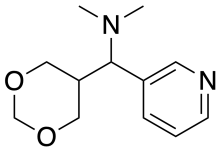
| Formula | C12H18N2O2 |
| Molar mass | 222.288 g·mol−1 |
| 3D model (JSmol) | |
SMILES
| |
InChI
| |
| | |
Doxpicomine (Doxpicodin, Doxpizodine) is a mild opioid analgesic drug.[1] The drug acts as a mu-opioid receptor agonist.[2] It is of fairly low potency, with a 400 mg dose of doxpicomine approximately equivalent in pain-killing effect to 8 mg morphine or 100 mg pethidine.[3][4] It has been used as a lead compound to derive further analogues, although all compounds in this family are comparatively weak mu agonists.[5]
References
- ↑ US patent 3905987, Booher RN, "m-Dioxane-5-Methylamine Analgesics", issued 09/16/1975, assigned to Eli Lilly
- ↑ Smits SE, Nickander R, Booher RN, Zimmerman DM, Wong DT, Hynes MD, Pohland A (February 1981). "Preclinical pharmacology of doxpicodin, a new analgesic". NIDA Research Monograph. 34: 75–81. PMID 6261137.
- ↑ Wang RI, Robinson N (June 1981). "Doxpicomine in postoperative pain". Clinical Pharmacology and Therapeutics. 29 (6): 771–5. doi:10.1038/clpt.1981.109. PMID 7014073. S2CID 24849960.
- ↑ Wang RI, Robinson N (January 1983). "Further efficacy evaluation of doxpicomine for postoperative pain". Journal of Clinical Pharmacology. 23 (1): 44–7. doi:10.1002/j.1552-4604.1983.tb02703.x. PMID 6341416. S2CID 36306783.
- ↑ Wünsch B, Bauschke G (March 1993). "[Benzomorphan analogs with doxpicomine partial structure: synthesis andpsychopharmacologic investigations of 5-aminomethyl- and 5-(alpha-aminobenzyl)- substituted 2,6-epoxy-3-benzoxocines]" [Benzomorphan analogs with doxpicomine partial structure: synthesis and psychopharmacologic investigations of 5-aminomethyl- and 5-(alpha-aminobenzyl)- substituted 2,6-epoxy-3-benzoxocines]. Archiv der Pharmazie (in German). 326 (3): 171–80. doi:10.1002/ardp.19933260311. PMID 8481096. S2CID 98749966.
This article is issued from Offline. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.