Estradiol/drospirenone
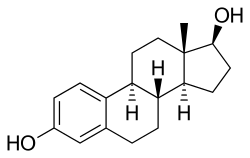 Estradiol | |
 Drospirenone | |
| Combination of | |
|---|---|
| Estradiol | Estrogen |
| Drospirenone | Progestogen; Progestin; Antimineralocorticoid; Antiandrogen |
| Clinical data | |
| Trade names | Angeliq |
| Other names | E2/DRSP; BAY-864891 |
| Routes of administration | By mouth |
| Drug class | Estrogen; Progestin; Progestogen; Antimineralocorticoid; Antiandrogen |
| Legal status | |
| Legal status |
|
| Identifiers | |
| CAS Number | |
Estradiol/drospirenone (E2/DRSP), sold under the brand name Angeliq, is a combination of estradiol (E2), an estrogen, and drospirenone (DRSP), a progestin, antimineralocorticoid, and antiandrogen, which is used in menopausal hormone therapy, specifically the treatment of menopausal syndrome and osteoporosis, in postmenopausal women.[1][2][3] It is taken by mouth and contains 0.5 to 1 mg E2 and 0.25 to 0.5 mg DRSP per tablet.[1] The medication was approved in the United States in 2005.[1] It is marketed widely throughout the world.[4]
See also
References
- 1 2 3 "ANGELIQ (drospirenone and estradiol) Prescribing Information" (PDF). Bayer HealthCare Pharmaceuticals Inc. U.S. Food and Drug Administration. February 2012.
- ↑ "Estradiol/Drospirenone - Bayer HealthCare Pharmaceuticals". AdisInsight.
- ↑ Rosano GM, Vitale C, Marazzi G, Volterrani M (February 2007). "Menopause and cardiovascular disease: the evidence". Climacteric : the Journal of the International Menopause Society. 10 (Suppl 1): 19–24. doi:10.1080/13697130601114917. PMID 17364594.
- ↑ "Drospirenone and Estradiol: Uses, Side Effects & Warnings".
External links
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
This article is issued from Offline. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.