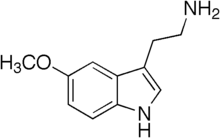
5-Methoxytryptamine
 | |
| Identifiers | |
|---|---|
IUPAC name
| |
| CAS Number | |
| PubChem CID | |
| IUPHAR/BPS | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEBI | |
| ChEMBL | |
| CompTox Dashboard (EPA) | |
| ECHA InfoCard | 100.009.231 |
| Chemical and physical data | |
| Formula | C11H14N2O |
| Molar mass | 190.246 g·mol−1 |
| 3D model (JSmol) | |
SMILES
| |
InChI
| |
| (verify) | |
5-Methoxytryptamine (5-MT), also known as mexamine, is a tryptamine derivative closely related to the neurotransmitters serotonin and melatonin. 5-MT has been shown to occur naturally in the body in low levels.[1] It is biosynthesized via the deacetylation of melatonin in the pineal gland.[1]
5-MT acts as a full agonist at the 5-HT1, 5-HT2, 5-HT4, 5-HT6, and 5-HT7 receptors.[2][3][4][5][6][7][8] It has no affinity for the 5-HT3 receptor and its affinity for the 5-HT1E receptor is very weak in comparison to the other 5-HT1 receptors.[5][9] Its affinity for the 5-HT5A receptor is unknown.
Measured affinity for some receptors (not a complete list):
- 5-HT1B receptors (Ki = 35 nM) [10]
- 5-HT1D receptors (Ki = 7.3 nM)[11]
- 5-HT1E receptors (Ki = 3151 nM)[12]
- 5-HT1F receptors (Ki = 1166 nM)[13]
- 5-HT2A receptors (Ki = 295 nM)[14]
- 5-HT2B receptors (Ki = 16.4 nM)[15]
- 5-HT2C receptors (Ki = 52.48 nM) [16]
- 5-HT4 receptors (Ki = 501.18 nM)[17]
- 5-HT6 receptors (Ki = 69.18 nM)[18]
- 5-HT7 receptors (Ki = 5.01 nM)[19]
See also
Indorenate
References
- 1 2 Galzin AM, Eon MT, Esnaud H, Lee CR, Pévet P, Langer SZ (1988). "Day-night rhythm of 5-methoxytryptamine biosynthesis in the pineal gland of the golden hamster (Mesocricetus auratus)". J. Endocrinol. 118 (3): 389–397. doi:10.1677/joe.0.1180389. PMID 2460575.
- ↑ Wu PH, Gurevich N, Carlen PL (1988). "Serotonin-1A receptor activation in hippocampal CA1 neurons by 8-hydroxy-2-(di-n-propylamino)tetralin, 5-methoxytryptamine and 5-hydroxytryptamine". Neurosci. Lett. 86 (1): 72–76. doi:10.1016/0304-3940(88)90185-1. PMID 2966313. S2CID 21620262.
- ↑ Yamada J, Sugimoto Y, Yoshikawa T, Horisaka K (1997). "Hyperglycemia induced by the 5-HT receptor agonist, 5-methoxytryptamine, in rats: involvement of the peripheral 5-HT2A receptor". Eur J Pharmacol. 323 (2–3): 235–240. doi:10.1016/S0014-2999(97)00029-0. PMID 9128844.
- ↑ Amemiya N, Hatta S, Takemura H, Ohshika H (1996). "Characterization of the contractile response induced by 5-methoxytryptamine in rat stomach fundus strips". Eur J Pharmacol. 318 (2–3): 403–409. doi:10.1016/S0014-2999(96)00777-7. PMID 9016931.
- 1 2 Craig DA, Eglen RM, Walsh LK, Perkins LA, Whiting RL, Clarke DE (1990). "5-Methoxytryptamine and 2-methyl-5-hydroxytryptamine-induced desensitization as a discriminative tool for the 5-HT3 and putative 5-HT4 receptors in guinea pig ileum". Naunyn Schmiedebergs Arch Pharmacol. 342 (1): 9–16. doi:10.1007/bf00178965. PMID 2402303. S2CID 24743785.
- ↑ Boess FG, Monsma Jr FJ, Carolo C, Meyer V, Rudler A, Zwingelstein C, Sleight AJ (1997). "Functional and radioligand binding characterization of rat 5-HT6 receptors stably expressed in HEK293 cells". Neuropharmacology. 36 (4–5): 713–720. doi:10.1016/S0028-3908(97)00019-1. PMID 9225298. S2CID 41813873.
- ↑ Hemedah M, Coupar IM, Mitchelson FJ (1999). "[3H]-Mesulergine labels 5-HT7 sites in rat brain and guinea-pig ileum but not rat jejunum". Br J Pharmacol. 126 (1): 179–188. doi:10.1038/sj.bjp.0702293. PMC 1565797. PMID 10051134.
- ↑ Glennon RA, Dukat M, Westkaemper RB (2000-01-01). "Serotonin Receptor Subtypes and Ligands". American College of Neurophyscopharmacology. Archived from the original on 21 April 2008. Retrieved 2008-04-11.
- ↑ Roth, Brian (2006). The serotonin receptors. Humana Press. p. 133. ISBN 978-1-58829-568-2.
- ↑ S. Nigra / Domenech T, et al., 1997
- ↑ Cortex / PEROUTKA ET AL., 1989
- ↑ Cloned / ZGOMBICK JM, ET AL., 1992
- ↑ Cloned / Adham N, et al., 1992
- ↑ Cortex / HOYER ET AL., 1987
- ↑ Cortex / WAINSCOTT DB, ET AL., 1996
- ↑ Cloned / BONHAUS DW, ET AL., 1997
- ↑ Caudate / ANSANAY H, ET AL.,1996
- ↑ Cloned / Hirst WD, et.al.,2003
- ↑ Cloned / BOESS FG, ET AL., 1994
This article is issued from Offline. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.