Dextromethorphan/bupropion
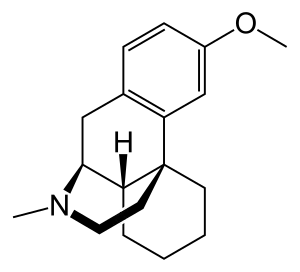 Dextromethorphan | |
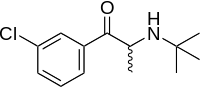 Bupropion | |
| Combination of | |
|---|---|
| Dextromethorphan | NMDA receptor antagonist, σ1 receptor agonist, serotonin-norepinephrine reuptake inhibitor, nicotinic acetylcholine receptor negative allosteric modulator, and other actions |
| Bupropion | Norepinephrine–dopamine reuptake inhibitor and nicotinic acetylcholine receptor negative allosteric modulator |
| Clinical data | |
| Trade names | Auvelity |
| Other names | DXM/BUP; AXS-05 |
| License data | |
| Routes of administration | By mouth |
| ATC code |
|
| Legal status | |
| Legal status | |
| Identifiers | |
| CAS Number | |
| KEGG | |
Dextromethorphan/bupropion (DXM/BUP), sold under the brand name Auvelity, is a combination medication for the treatment of major depressive disorder (MDD).[1] Its active components are dextromethorphan (DXM) and bupropion.[1] Patients who stayed on the medication had an average of 11% greater reduction in depressive symptoms than placebo in an FDA approval trial.[2][3] It is taken as a tablet by mouth.[1]
Side effects of dextromethorphan/bupropion include dizziness, headache, diarrhea, somnolence, dry mouth, sexual dysfunction, and hyperhidrosis, among others.[1] The mechanism of action of dextromethorphan/bupropion in the treatment of depression is unknown.[1]
Dextromethorphan/bupropion was developed by Axsome Therapeutics and was approved for the treatment of major depressive disorder in the United States in August 2022.[1]
Medical uses
Depression
_versus_placebo_in_the_GEMINI_trial.png.webp)
Dextromethorphan/bupropion is approved for the treatment of major depressive disorder.[1] Dextromethorphan and bupropion have both individually been reported to be effective for the treatment of this condition.[6][7][8] The effect size of bupropion alone relative to placebo for depression is small,[7][8] whereas only limited evidence exists for dextromethorphan alone.[6] The combination was approved in the US on the basis of two regulatory clinical trials.[1]
In Study 1 (GEMINI), a 6-week randomized controlled trial of dextromethorphan/bupropion versus placebo in people with major depressive disorder, scores on the Montgomery–Åsberg Depression Rating Scale (MADRS)—a scale with a range of 0 to 60 points—decreased with dextromethorphan/bupropion by 15.9 points from a baseline score of 33.6 points (an approximate 47% reduction) and decreased with placebo by 12.1 points from a baseline score of 33.2 points (an approximate 36% reduction).[1][3] This resulted in a least-squares mean difference in reduction of depression scores between dextromethorphan/bupropion and placebo of 3.9 points, with the placebo group showing approximately 76% of the improvement in depression scores as the dextromethorphan/bupropion group and with depression scores at baseline improving overall about 11% more with the medication than with placebo.[1][3] In antidepressant trials of 6 to 8 weeks duration recorded in the Food and Drug Administration (FDA) database, the average difference from placebo with other antidepressants was 2.5 points.[3] The mean improvement in scores with dextromethorphan/bupropion was statistically significant but not clinically significant[9] relative to placebo at all assessed timepoints including at the end of week 1, although at the end of the study some patients did have clinically significant improvement.[1][3]
In Study 2 (STRIDE-1), dextromethorphan/bupropion was compared with bupropion alone in another randomized controlled trial.[1] The dose of bupropion in the study was lower than the target dose recommended for clinical practice.[10] In this study, dextromethorphan/bupropion showed significantly greater improvement than bupropion alone in the first two weeks of treatment but not by week 6 of treatment in people with major depressive disorder.[1][11] The baseline scores were 33.4 points with dextromethorphan/placebo and 33.2 points with placebo, while the score reductions at week 1 were 5.2 points on the MADRS with dextromethorphan/bupropion and 3.6 points with bupropion (a 1.6-point difference), at week 2 were 8.0 points with dextromethorphan/bupropion and 6.1 points with bupropion (a 1.9-point difference), and at week 6 were 11.6 points with dextromethorphan/bupropion and 9.4 points with bupropion (a 2.2-point difference).[11][12] On the basis of this trial, the FDA concluded that dextromethorphan contributes to the apparent antidepressant effects of dextromethorphan/bupropion.[1]
Side effects
Side effects of dextromethorphan/bupropion include dizziness, nausea, headache, diarrhea, somnolence, dry mouth, sexual dysfunction (including abnormal orgasm, erectile dysfunction, decreased libido, and anorgasmia), hyperhidrosis, anxiety, constipation, decreased appetite, insomnia, arthralgia, fatigue, paresthesia, and blurred vision.[1] These side effects occurred at rates ≥2% and to a greater extent than with placebo in clinical trials.[1]
Pharmacology
Pharmacodynamics
Dextromethorphan acts as an NMDA receptor antagonist, σ1 receptor agonist, and serotonin–norepinephrine reuptake inhibitor, among other actions, while bupropion acts as a norepinephrine–dopamine reuptake inhibitor and nicotinic acetylcholine receptor negative allosteric modulator.[1][13] Bupropion is also a potent inhibitor of CYP2D6, and thereby inhibits the metabolism of dextromethorphan.[13] Dextromethorphan/bupropion has less activity as an NMDA receptor antagonist than dextromethorphan alone.[11] This is because bupropion is a potent CYP2D6 inhibitor and prevents the bioactivation of dextromethorphan into dextrorphan, a much more potent NMDA receptor antagonist and weaker serotonin reuptake inhibitor than dextromethorphan itself.[11] The mechanism of action of dextromethorphan/bupropion in the treatment of depression is unknown, although the preceding pharmacological actions are assumed to be involved.
Pharmacokinetics
When administered together as dextromethorphan/bupropion, the elimination half-life of dextromethorphan is 22 hours and the elimination half-life of bupropion is 15 hours.[1] The elimination half-lives of bupropion active metabolites are 35 hours for hydroxybupropion, 44 hours for erythrohydrobupropion, and 33 hours for threohydrobupropion.[1] Bupropion inhibits the metabolism of dextromethorphan by inhibiting the enzyme CYP2D6, the major enzyme responsible for the metabolism of dextromethorphan.[1] This in turn improves the bioavailability of dextromethorphan, prolongs its half-life, prevents its metabolism into dextrorphan, and increases the ratio of dextromethorphan to dextrorphan in the body.[1][13][14][6][15]
History
Dextromethorphan/bupropion was developed by Axsome Therapeutics.[16] It was approved for the treatment of major depressive disorder by the US Food and Drug Administration in August 2022.[1]
Society and culture
Brand names
Dextromethorphan/bupropion is sold under the brand name Auvelity.[1]
Legal status
Dextromethorphan/bupropion is not a controlled substance in the United States.[1] The misuse potential of dextromethorphan and bupropion has not been systematically studied.[1] However, both dextromethorphan and bupropion may have misuse liability at supratherapeutic doses.[1][17][18][19] Despite the known misuse potential of dextromethorphan, it is available widely as an over-the-counter drug.[18] Conversely, bupropion is a prescription-only medication.[20]
Research
Dextromethorphan/bupropion is under development for the treatment of agitation in Alzheimer's disease and smoking withdrawal.[16][21][22] As of August 2022, it is in phase III clinical trials for agitation and phase II trials for smoking withdrawal.[16]
References
- 1 2 3 4 5 6 7 8 9 10 11 12 13 14 15 16 17 18 19 20 21 22 23 24 25 26 27 28 "Auvelity- dextromethorphan hydrobromide, bupropion hydrochloride tablet, multilayer, extended release". DailyMed. 15 December 2022. Retrieved 21 January 2023.
- 1 2 "AUVELITY (dextromethorphan hydrobromide and bupropion hydrochloride) extended-release tablets, for oral use" (PDF). October 2022.
- 1 2 3 4 5 6 Iosifescu DV, Jones A, O'Gorman C, Streicher C, Feliz S, Fava M, et al. (May 2022). "Efficacy and Safety of AXS-05 (Dextromethorphan-Bupropion) in Patients With Major Depressive Disorder: A Phase 3 Randomized Clinical Trial (GEMINI)". J Clin Psychiatry. 83 (4). doi:10.4088/JCP.21m14345. PMID 35649167. S2CID 249104681.
- 1 2 O'Gorman C, Jones A, Thomas Z, Iosifescu DV, Tabuteau H (June 2021). "W19 Rapid Effects of AXS-05, an Oral NMDA Receptor Antagonist, in Major Depressive Disorder: Results from Two Randomized, Double-Blind, Controlled Trials" (PDF). American Society of Clinical Psychopharmacology, Annual Meeting 2021, 1-4 June 2021 (PDF).
- 1 2 O'Gorman C, Feliz S, Jones A, Streicher C, Thomas Z, Tabuteau H (June 2021). "W20 Effects of AXS-05, an Oral NMDA Receptor Antagonist with Multimodal Activity, on Patient Reported Depressive Symptoms in Major Depressive Disorder: Results from the GEMINI Phase 3 Double-Blind, Placebo-Controlled Trial" (PDF) (PDF).
- 1 2 3 Majeed A, Xiong J, Teopiz KM, Ng J, Ho R, Rosenblat JD, et al. (March 2021). "Efficacy of dextromethorphan for the treatment of depression: a systematic review of preclinical and clinical trials". Expert Opin Emerg Drugs. 26 (1): 63–74. doi:10.1080/14728214.2021.1898588. ISSN 1472-8214. PMID 33682569. S2CID 232141396.
- 1 2 Cipriani A, Furukawa TA, Salanti G, Chaimani A, Atkinson LZ, Ogawa Y, Leucht S, Ruhe HG, Turner EH, Higgins JP, Egger M, Takeshima N, Hayasaka Y, Imai H, Shinohara K, Tajika A, Ioannidis JP, Geddes JR (April 2018). "Comparative efficacy and acceptability of 21 antidepressant drugs for the acute treatment of adults with major depressive disorder: a systematic review and network meta-analysis". Lancet. 391 (10128): 1357–1366. doi:10.1016/S0140-6736(17)32802-7. PMC 5889788. PMID 29477251.
- 1 2 Monden R, Roest AM, van Ravenzwaaij D, Wagenmakers EJ, Morey R, Wardenaar KJ, de Jonge P (August 2018). "The comparative evidence basis for the efficacy of second-generation antidepressants in the treatment of depression in the US: A Bayesian meta-analysis of Food and Drug Administration reviews". J Affect Disord. 235: 393–398. doi:10.1016/j.jad.2018.04.040. PMID 29677603. S2CID 5011570.
- ↑ Turkoz I, Alphs L, Singh J, Jamieson C, Daly E, Shawi M, Sheehan J, Trivedi M, Rush A (2021). "Clinically meaningful changes on depressive symptom measures and patient‐reported outcomes in patients with treatment‐resistant depression". Acta Psychiatrica Scandinavica. 143 (3): 253–263. doi:10.1111/acps.13260. PMC 7986932. PMID 33249552.
- ↑ Fava M, Rush A, Thase M, Clayton A, Stahl S, Pradko J, Johnston J (2005). "15 Years of Clinical Experience With Bupropion HCl: From Bupropion to Bupropion SR to Bupropion XL". The Primary Care Companion to the Journal of Clinical Psychiatry. 7 (3): 106–113. doi:10.4088/pcc.v07n0305. PMC 1163271. PMID 16027765.
- 1 2 3 4 Sakurai H, Yonezawa K, Tani H, Mimura M, Bauer M, Uchida H (July 2022). "Novel Antidepressants in the Pipeline (Phase II and III): A Systematic Review of the US Clinical Trials Registry". Pharmacopsychiatry. 55 (4): 193–202. doi:10.1055/a-1714-9097. PMC 9259184. PMID 35045580.
- ↑ Axsome Therapeutics (30 March 2020). "STRIDE-1 Phase 3 Trial of AXS-05 in TRD Topline Results Conference Call" (PDF).
- 1 2 3 Jefferson JW, Pradko JF, Muir KT (November 2005). "Bupropion for major depressive disorder: Pharmacokinetic and formulation considerations". Clin Ther. 27 (11): 1685–95. doi:10.1016/j.clinthera.2005.11.011. PMID 16368442.
- ↑ Stahl SM (October 2019). "Dextromethorphan/Bupropion: A Novel Oral NMDA (N-methyl-d-aspartate) Receptor Antagonist with Multimodal Activity". CNS Spectr. 24 (5): 461–466. doi:10.1017/S1092852919001470. PMID 31566163. S2CID 203607617.
- ↑ Schoedel KA, Morrow SA, Sellers EM (2014). "Evaluating the safety and efficacy of dextromethorphan/quinidine in the treatment of pseudobulbar affect". Neuropsychiatr Dis Treat. 10: 1161–74. doi:10.2147/NDT.S30713. PMC 4079824. PMID 25061302.
- 1 2 3 "Bupropion/dextromethorphan". Adis Insight. Archived from the original on 21 September 2021. Retrieved 16 December 2019.
- ↑ Silva AR, Dinis-Oliveira RJ (May 2020). "Pharmacokinetics and pharmacodynamics of dextromethorphan: clinical and forensic aspects". Drug Metab Rev. 52 (2): 258–282. doi:10.1080/03602532.2020.1758712. PMID 32393072. S2CID 218599441.
- 1 2 Stanciu CN, Penders TM, Rouse EM (August 2016). "Recreational use of dextromethorphan, "Robotripping"-A brief review". Am J Addict. 25 (5): 374–7. doi:10.1111/ajad.12389. PMID 27288091.
- ↑ Costa R, Oliveira NG, Dinis-Oliveira RJ (August 2019). "Pharmacokinetic and pharmacodynamic of bupropion: integrative overview of relevant clinical and forensic aspects". Drug Metab Rev. 51 (3): 293–313. doi:10.1080/03602532.2019.1620763. PMID 31124380. S2CID 163167323.
- ↑ "Bupropion: Drug Uses, Dosage, Side Effects".
- ↑ Wilkinson ST, Sanacora G (February 2019). "A new generation of antidepressants: an update on the pharmaceutical pipeline for novel and rapid-acting therapeutics in mood disorders based on glutamate/GABA neurotransmitter systems". Drug Discovery Today. 24 (2): 606–615. doi:10.1016/j.drudis.2018.11.007. PMC 6397075. PMID 30447328.
- ↑ "Axsome depression drug meets late-stage study goal, shares soar 56%". Reuters. 16 December 2019. Archived from the original on 16 December 2019. Retrieved 16 December 2019.