Alnespirone
 | |
| Clinical data | |
|---|---|
| ATC code |
|
| Identifiers | |
IUPAC name
| |
| CAS Number | |
| PubChem CID | |
| ChemSpider |
|
| UNII | |
| ChEMBL | |
| CompTox Dashboard (EPA) | |
| Chemical and physical data | |
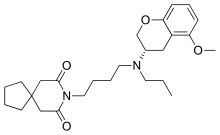
| Formula | C26H38N2O4 |
| Molar mass | 442.600 g·mol−1 |
| 3D model (JSmol) | |
SMILES
| |
InChI
| |
| (verify) | |
Alnespirone (S-20,499) is a selective 5-HT1A receptor full agonist of the azapirone chemical class.[1][2][3] It has antidepressant and anxiolytic effects.[1]
See also
References
- 1 2 Griebel G, Misslin R, Pawlowski M, Guardiola Lemaître B, Guillaumet G, Bizot-Espiard J (1992). "Anxiolytic-like effects of a selective 5-HT1A agonist, S20244, and its enantiomers in mice". NeuroReport. 3 (1): 84–86. doi:10.1097/00001756-199201000-00022. PMID 1351756.
- ↑ Simon P, Guardiola B, Bizot-Espiard J, Schiavi P, Costentin J (1992). "5-HT1A receptor agonists prevent in rats the yawning and penile erections induced by direct dopamine agonists". Psychopharmacology. 108 (1–2): 47–50. doi:10.1007/BF02245284. PMID 1357709. S2CID 22385029.
- ↑ Astier B, Lambás Señas L, Soulière F, Schmitt P, Urbain N, Rentero N, Bert L, Denoroy L, Renaud B, Lesourd M, Muñoz C, Chouvet G (2003). "In vivo comparison of two 5-HT1A receptors agonists alnespirone (S-20499) and buspirone on locus coeruleus neuronal activity". Eur J Pharmacol. 459 (1): 17–26. doi:10.1016/S0014-2999(02)02814-5. PMID 12505530.
| 5-HT1AR agonists | |
|---|---|
| GABAAR PAMs |
|
| Gabapentinoids (α2δ VDCC blockers) | |
| Antidepressants |
|
| Sympatholytics (Antiadrenergics) |
|
| Others | |
| |
Serotonin receptor modulators | |||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 5-HT1 |
| ||||||||||||||||||||||||||||||||||||||
| 5-HT2 |
| ||||||||||||||||||||||||||||||||||||||
| 5-HT3–7 |
| ||||||||||||||||||||||||||||||||||||||
| |||||||||||||||||||||||||||||||||||||||
| Simple piperazines (no additional rings) |
|
|---|---|
| Phenylpiperazines |
|
| Benzylpiperazines | |
| Diphenylalkylpiperazines (benzhydrylalkylpiperazines) |
|
| Pyrimidinylpiperazines |
|
| Pyridinylpiperazines |
|
| Benzo(iso)thiazolylpiperazines | |
| Tricyclics (piperazine attached via side chain) |
|
| Others/Uncategorized |
|
This article is issued from Offline. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.