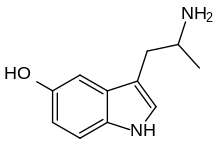
alpha-Methylserotonin
α-Methylserotonin (αMS), also known as α-methyl-5-hydroxytryptamine (α-methyl-5-HT) or 5-hydroxy-α-methyltryptamine (5-HO-αMT), is a tryptamine derivative closely related to the neurotransmitter serotonin (5-HT). It acts as a non-selective serotonin receptor agonist and has been used extensively in scientific research to study the function of the serotonin system.[1]
 | |
 | |
| Clinical data | |
|---|---|
| Routes of administration | Oral |
| ATC code |
|
| Legal status | |
| Legal status |
|
| Identifiers | |
IUPAC name
| |
| CAS Number |
|
| PubChem CID | |
| IUPHAR/BPS | |
| ChemSpider |
|
| UNII | |
| ChEBI | |
| ChEMBL | |
| CompTox Dashboard (EPA) | |
| Chemical and physical data | |
| Formula | C11H14N2O |
| Molar mass | 190.246 g·mol−1 |
| 3D model (JSmol) | |
SMILES
| |
InChI
| |
| (verify) | |
Unlike serotonin, αMS is not metabolized by monoamine oxidase on account of the α-methyl substituent blocking the enzyme's access to the amine. As a result, it has a much longer half-life in comparison.[2][3] Similarly to serotonin however, αMS poorly crosses the blood-brain-barrier due to its free hydroxyl group, and thus has only weak or no central effects when administered peripherally.[2]
α-Methyltryptophan (αMTP) is a prodrug to αMS which does cross the blood-brain-barrier and thus efficiently delivers αMS into the central nervous system.[4][5] As a result, αMTP acts as an orally bioavailable false or substitute neurotransmitter for serotonin, and has been suggested as a possible therapeutic agent in the treatment of disorders where serotonin is deficient.[4][5] The O-methylated analogue of αMS, 5-MeO-αMT, also readily enters the brain and could be used for this purpose as well.[2][6]
Legal status
United States
5-Hydroxy-alpha-methyltryptamine is not scheduled at the federal level in the United States,[7] but it could be considered an analog of alpha-methyltryptamine (AMT), in which case, purchase, sales, or possession could be prosecuted under the Federal Analog Act.
Florida
5-Hydroxy-alpha-methyltryptamine is a Schedule I controlled substance in the state of Florida making it illegal to buy, sell, or possess in Florida.[8]
See also
- 2-Methyl-5-hydroxytryptamine
- 5-Carboxamidotryptamine
- 5-Methoxytryptamine
- α-Methyldopamine
- Serotonin
References
- Ismaiel AM, Titeler M, Miller KJ, Smith TS, Glennon RA (February 1990). "5-HT1 and 5-HT2 binding profiles of the serotonergic agents alpha-methylserotonin and 2-methylserotonin". Journal of Medicinal Chemistry. 33 (2): 755–8. doi:10.1021/jm00164a046. PMID 2299641.
- "Erowid Online Books : "TIHKAL" - #48 a-MT".
- Diksic M, Nagahiro S, Sourkes TL, Yamamoto YL (January 1990). "A new method to measure brain serotonin synthesis in vivo. I. Theory and basic data for a biological model". Journal of Cerebral Blood Flow and Metabolism. 10 (1): 1–12. doi:10.1038/jcbfm.1990.2. PMID 2298826.
- Sourkes TL, Montine TJ, Missala K (1990). "Alpha-methylserotonin, a substitute transmitter for serotonergic neurons". Progress in Neuro-psychopharmacology & Biological Psychiatry. 14 (5): 829–32. doi:10.1016/0278-5846(90)90055-l. PMID 1705718. S2CID 25604266.
- Sourkes TL (1991). "Alpha-methyltryptophan as a therapeutic agent". Progress in Neuro-psychopharmacology & Biological Psychiatry. 15 (6): 935–8. doi:10.1016/0278-5846(91)90020-2. PMID 1763198. S2CID 31504647.
- "Erowid Online Books : "TIHKAL" - #5. a,O-DMS".
- §1308.11 Schedule I.
- Florida Statutes - Chapter 893 - DRUG ABUSE PREVENTION AND CONTROL
Serotonin receptor modulators | |||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 5-HT1 |
| ||||||||||||||||||||||||||||||||||||||
| 5-HT2 |
| ||||||||||||||||||||||||||||||||||||||
| 5-HT3–7 |
| ||||||||||||||||||||||||||||||||||||||
| |||||||||||||||||||||||||||||||||||||||
|