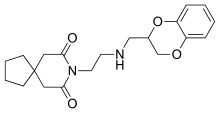
Binospirone
Binospirone (MDL-73,005-EF) is a drug which acts as a partial agonist at 5-HT1A somatodendritic autoreceptors but as an antagonist at postsynaptic 5-HT1A receptors.[1] It has anxiolytic effects.[2]
 | |
 | |
| Clinical data | |
|---|---|
| ATC code |
|
| Identifiers | |
IUPAC name
| |
| CAS Number |
|
| PubChem CID | |
| ChemSpider | |
| UNII |
|
| ChEMBL | |
| Chemical and physical data | |
| Formula | C20H26N2O4 |
| Molar mass | 358.438 g·mol−1 |
| 3D model (JSmol) | |
SMILES
| |
InChI
| |
| (verify) | |
Synthesis
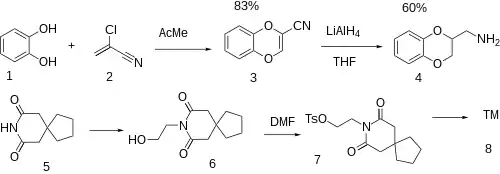
The reaction between catechol (1) and 2-chloroacrylonitrile [920-37-6] (2) gives 1,4-Benzodioxin-2-Carbonitrile [91889-45-1] (3). The reduction of the nitrile group with lithium aluminium hydride gives 2,3-dihydro-1,4-benzodioxin-3-ylmethanamine [4442-59-5] (4).
Alkylation of 3,3-Tetramethyleneglutarimide [1075-89-4] (5) with (for example) 2-chloroethanol gives 8-(2-hydroxyethyl)-8-azaspiro[4.5]decane-7,9-dione [21098-04-4] (6). FGI of the alcohol to a better leaving group by reaction with tosyl chloride gives CID:14839216 (7).
Convergent synthesis now completes the synthesis of Binospirone (8).
See also
References
- Bertrand F, Lehmann O, Galani R, Lazarus C, Jeltsch H, Cassel JC (April 2001). "Effects of MDL 73005 on water-maze performances and locomotor activity in scopolamine-treated rats". Pharmacology, Biochemistry, and Behavior. 68 (4): 647–60. doi:10.1016/S0091-3057(01)00448-8. PMID 11526961. S2CID 8595441.
- Moser PC, Tricklebank MD, Middlemiss DN, Mir AK, Hibert MF, Fozard JR (February 1990). "Characterization of MDL 73005EF as a 5-HT1A selective ligand and its effects in animal models of anxiety: comparison with buspirone, 8-OH-DPAT and diazepam". British Journal of Pharmacology. 99 (2): 343–9. doi:10.1111/j.1476-5381.1990.tb14706.x. PMC 1917389. PMID 1970269.
- Marcel Hibert, Maurice W. Gittos, U.S. Patent 4,612,312 (1986 to Merrell Dow Pharmaceuticals Inc.).
| 5-HT1AR agonists | |
|---|---|
| GABAAR PAMs |
|
| Gabapentinoids (α2δ VDCC blockers) | |
| Antidepressants |
|
| Sympatholytics (Antiadrenergics) |
|
| Others | |
| |
Serotonin receptor modulators | |||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 5-HT1 |
| ||||||||||||||||||||||||||||||||||||||
| 5-HT2 |
| ||||||||||||||||||||||||||||||||||||||
| 5-HT3–7 |
| ||||||||||||||||||||||||||||||||||||||
| |||||||||||||||||||||||||||||||||||||||
| Simple piperazines (no additional rings) |
|
|---|---|
| Phenylpiperazines |
|
| Benzylpiperazines | |
| Diphenylalkylpiperazines (benzhydrylalkylpiperazines) |
|
| Pyrimidinylpiperazines |
|
| Pyridinylpiperazines |
|
| Benzo(iso)thiazolylpiperazines | |
| Tricyclics (piperazine attached via side chain) |
|
| Others/Uncategorized |
|