Enilospirone
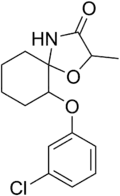
Enilospirone (CERM-3,726) is a selective 5-HT1A receptor agonist of the azapirone class.[1]
 | |
| Clinical data | |
|---|---|
| Routes of administration | Oral |
| ATC code |
|
| Legal status | |
| Legal status |
|
| Identifiers | |
IUPAC name
| |
| CAS Number | |
| PubChem CID | |
| ChemSpider | |
| UNII | |
| ECHA InfoCard | 100.056.284 |
| Chemical and physical data | |
| Formula | C15H18ClNO3 |
| Molar mass | 295.76 g·mol−1 |
Synthesis
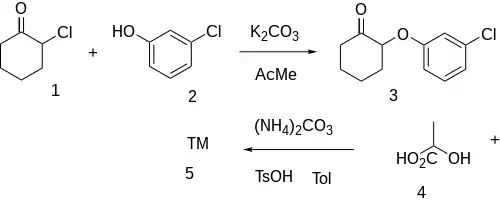
Patent:[2]
Base catalyzed Williamson ether synthesis between 2-chlorocyclohexanone [822-87-7] (1) and 3-Chlorophenol [108-43-0] (2) gives 2-(3-Chlorophenoxy)cyclohexan-1-one [59798-91-3] (3). Condensation with lactic acid (4) and ammonium carbonate gives Enilospirone (5).
See also
References
- Maurel JL, Autin JM, Funes P, Newman-Tancredi A, Colpaert F, Vacher B (October 2007). "High-efficacy 5-HT1A agonists for antidepressant treatment: a renewed opportunity". Journal of Medicinal Chemistry. 50 (20): 5024–33. doi:10.1021/jm070714l. PMID 17803293.
- DE2542154 idem US4000292 idem Roland Yves Mauvernay et al. U.S. Patent 4,086,352 (1978 to Centre Europeen De Recherches Mauvernay).
Further reading
- Nicholson AN, Stone BM (1982). "6-(3-chloro)-phenoxy-2-methyl-1-oxa-4-azospiro-[4,5]decane-3-one (CERM 3726) and sleep of healthy men". Psychopharmacology. 76 (2): 157–9. doi:10.1007/BF00435270. PMID 6805026. S2CID 666391.
| 5-HT1AR agonists | |
|---|---|
| GABAAR PAMs |
|
| Gabapentinoids (α2δ VDCC blockers) | |
| Antidepressants |
|
| Sympatholytics (Antiadrenergics) |
|
| Others | |
| |
Serotonin receptor modulators | |||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 5-HT1 |
| ||||||||||||||||||||||||||||||||||||||
| 5-HT2 |
| ||||||||||||||||||||||||||||||||||||||
| 5-HT3–7 |
| ||||||||||||||||||||||||||||||||||||||
| |||||||||||||||||||||||||||||||||||||||
| Simple piperazines (no additional rings) |
|
|---|---|
| Phenylpiperazines |
|
| Benzylpiperazines | |
| Diphenylalkylpiperazines (benzhydrylalkylpiperazines) |
|
| Pyrimidinylpiperazines |
|
| Pyridinylpiperazines |
|
| Benzo(iso)thiazolylpiperazines | |
| Tricyclics (piperazine attached via side chain) |
|
| Others/Uncategorized |
|
This article is issued from Wikipedia. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.