25I-NBOMe
 | |
 | |
| Clinical data | |
|---|---|
| Routes of administration | Buccal (sublabial), sublingual, insufflated, inhalation, intravenous, intramuscular, rectal |
| ATC code |
|
| Legal status | |
| Legal status |
|
| Identifiers | |
IUPAC name
| |
| CAS Number |
|
| PubChem CID | |
| ChemSpider | |
| UNII |
|
| ChEMBL | |
| CompTox Dashboard (EPA) | |
| Chemical and physical data | |
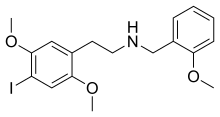
| Formula | C18H22INO3 |
| Molar mass | 427.282 g·mol−1 |
| 3D model (JSmol) | |
SMILES
| |
InChI
| |
| | |
| Part of a series on |
| Psychedelia |
|---|
25I-NBOMe (2C-I-NBOMe, Cimbi-5, Smiles and also shortened to "25I") is a synthetic hallucinogen that is used in biochemistry research for mapping the brain's usage of the type 2A serotonin receptor; it is also sometimes used for recreational purposes. A derivative of the substituted phenethylamine 2C-I family, it is the most well-known member of the 25-NB family. It was discovered in 2003 by chemist Ralf Heim at the Free University of Berlin, who published his findings in his PhD dissertation.[2] The compound was subsequently investigated by a team at Purdue University led by David Nichols.[3]
The carbon-11 labelled version of 25I-NBOMe, [11C]Cimbi-5, was synthesized and validated as a radiotracer for positron emission tomography (PET) in Copenhagen.[4][5] Being the first 5-HT2A receptor full agonist PET radioligand, [11C]-CIMBI-5 shows promise as a more functional marker of these receptors, particularly in their high affinity states.[4]
Street and media nicknames for this drug are: "N-Bomb", "Solaris", "Smiles", and "Wizard".[6][7][8]
Recreational use
Although 25I-NBOMe was discovered in 2003, it did not emerge as a common recreational drug until 2010, when it was first sold by vendors specializing in the supply of designer drugs.[9] In a slang context, the name of the compound is often shortened to "25I" or is simply called "N-Bomb".[10] According to a 2014 survey, 25I-NBOMe was the most frequently used of the NBOMe series.[11] By 2013, case reports of 25I-NBOMe intoxication, with and without analytic confirmation of the drug in the body, were becoming increasingly common in the medical literature.[12]
25I-NBOMe is widely rumored to be orally inactive; however, apparent overdoses have occurred via oral administration. Common routes of administration include sublingual, buccal, and intranasal.[11] For sublingual and buccal administration, 25I-NBOMe is often applied to sheets of blotter paper of which small portions (tabs) are held in the mouth to allow absorption through the oral mucosa.[13][14] There are reports of intravenous injection of 25I-NBOMe solution and smoking the drug in powdered form.[15][16]
Due to its potency and much lower cost than so-called classical or traditional psychedelics, 25I-NBOMe blotters are sometimes misrepresented as, or mistaken for, LSD blotters.[17] Even small quantities of 25I-NBOMe can produce a large number of blotters. Vendors would import 25I-NBOMe in bulk (e.g. 1 kg containers) and resell individual doses for a considerable profit.[14]
Dosage
25I-NBOMe is potent, being active in sub-milligram doses. A common dose of the hydrochloride salt is 600–1,200 µg. The UK Advisory Council on the Misuse of Drugs states that a common dose is between 50 and 100 µg,[14] although other sources indicate that these figures are incorrect; Erowid tentatively suggests that the threshold dosage for humans is 50–250 µg, with a light dose between 200–600 µg, a common dose at 500–800 µg, and a strong dose at 700–1500 µg.[18] At this level of potency, it is not possible to accurately measure a single dose of 25I-NBOMe powder without an analytical balance, and attempting to do so may put the user at significant risk of overdose.[14]
Effects
25I-NBOMe effects usually last 6–10 hours if taken sublingually, or buccally (between gum and cheek).[16] When it is insufflated (snorted), effects usually last 4–6 hours.[16]
25I-NBOMe has similar effects to LSD, though users report more negative effects while under the influence and more risk of harm following use as compared to the classical psychedelics.[11]
Case reports of seven British males who presented to an emergency room following analytically confirmed 25I-NBOMe intoxication suggest the following potential adverse effects: "tachycardia (n = 7), hypertension (4), agitation (6), aggression, visual and auditory hallucinations (6), seizures (3), hyperpyrexia (3), clonus (2), elevated white cell count (2), elevated creatine kinase (7), metabolic acidosis (3), and acute kidney injury (1)."[15]
25I-NBOMe can be consumed in liquid, powder or paper form and can be snorted, injected, mixed with food, or smoked, but sublingual administration is most common.[19]
Toxicity
Recreational use of 25I-NBOMe carries a significant risk of both pharmacological and behavioral toxicity.[12][15] 25I-NBOMe is a relatively new substance, and little is known about its pharmacological risks or its interaction with other substances. The LD50 has not yet been determined.[20] It is a highly potent serotonin agonist and, due to its psychedelic effects and ambiguous legal status, a designer drug with reports of recreational use beginning in 2010. Reports of deaths and significant injuries have been attributed to the use of 25I-NBOMe, prompting some governments to control its possession, production, and sale. The website Erowid states that 25I-NBOMe is extremely potent and should not be snorted, and that the drug "appears to have led to several deaths in the past year."[17] Several non-fatal overdoses requiring prolonged hospitalization have also been reported.[14][12][15]
As of August 2015, 25I-NBOMe has reportedly led to at least 19 overdose deaths in the United States.[10][21] In June 2012, two teens in Grand Forks, North Dakota and East Grand Forks, Minnesota fatally overdosed on a substance that was allegedly 25I-NBOMe, resulting in lengthy sentences for two of the parties involved and a Federal indictment against the Texas-based online vendor.[22] A 21-year-old man from Little Rock, Arkansas died in October 2012 after taking a liquid drop of the drug nasally at a music festival. He was reported to have consumed caffeinated alcoholic beverages for "several hours" beforehand. It is unclear what other drugs he may have consumed, as autopsies generally do not test for the presence of research chemicals.[23] In January 2013, an 18-year-old in Scottsdale, Arizona, died after consuming 25I-NBOMe sold as LSD; a toxicology screening found no other drugs in the person's system. The drug is the suspected cause of death in another Scottsdale, Arizona, incident in April 2013.[14] It is also cited in the death of a 21-year-old woman in August 2013[24] and the death of a 17-year-old in Minnesota in January 2014,[25] as well as the death of a 15-year old in Washington in September 2014.[26]
25I-NBOMe has been implicated in multiple deaths in Australia. In March 2012, a man in Australia died from injuries sustained by running into trees and power poles while intoxicated by 25I-NBOMe.[27]
25I-NBOMe has been linked to a major case on January 20, 2016 in Cork, Republic of Ireland, which left six teenagers hospitalized, one of whom later died. At least one of the teenagers suffered a cardiac arrest, according to reports, along with extreme internal bleeding.[28]
25I-NBOMe presumably exhibits functional selectivity at the 5HT2A receptor similar to other phenethylamine hallucinogens, activating the Phospholipase A2 signal cascade,[29] which is responsible for the release of Thromboxane A2, triggering blood platelet aggregation. Excessive concentrations of TXA2 could lead to thrombosis, which when coupled with 25I-NBOMe's vasoconstrictive effect, is a major risk factor for cardiac ischemia, a dangerous condition the symptoms of which are present in several toxicological reports.[20]
Pharmacology
| Receptor | Kd (nM) | ± |
|---|---|---|
| 5-HT2A | 0.044 | |
| 5-HT2B | 231 | 73 |
| 5-HT2C | 2 | |
| 5-HT6 | 73 | 12 |
| μ-opioid | 82 | 14 |
| κ-opioid | 288 | 50 |
| H1 | 189 | 35 |
25I-NBOMe acts as a highly potent full agonist for the human 5-HT2A receptor,[30][32] with a dissociation constant (Kd) of 0.044 nM, making it some sixteen times the potency of 2C-I itself at this receptor. A radiolabelled form of 25I-NBOMe can be used for mapping the distribution of 5-HT2A receptors in the brain.[31]
25I-NBOMe induces a head-twitch response in mice which is blocked completely by a selective 5-HT2A antagonist, suggesting its psychedelic effects are mediated by 5-HT2A. This study suggested that 25I-NBOMe is approximately 14-fold more potent than 2C-I in-vivo.[33]
While in-vitro studies showed that N-benzyl derivatives of 2C-I were significantly increased in potency compared to 2C-I, the N-benzyl derivatives of the related compound DOI were inactive.[34]
25I-NBOMe also has weaker interactions with multiple other receptors. Kd values for interaction with the following targets were greater than 500 nM: 5-HT1A, D3, H2, 5-HT1D, α1A adrenergic, δ opioid, serotonin uptake transporter, 5-HT5A, 5-HT1B, D2, 5-HT7, D1, 5-HT3, 5-HT1E, D5, muscarinic M1-M5, H3, and the dopamine uptake transporter.[31]
Chemistry
Like other 2C-X-NBOMe molecules, 25I-NBOMe is a derivative of the 2C family of phenethylamines described by chemist Alexander Shulgin in his book PiHKAL.[14][12] Specifically, 25I-NBOMe is an N-benzyl derivative of the phenethylamine molecule 2C-I, formed by adding a 2-methoxybenzyl (BnOMe) onto the nitrogen (N) of the phenethylamine backbone. This substitution significantly increases the potency of the molecule.[14]
Analogues
Analogues and derivatives of 2C-I:
25I-NB*:
- 25I-NBF
- 25I-NBMD
- 25I-NB34MD
- 25I-NBOH
- 25I-NBOMe (NBOMe-2CI)
- 25I-NB3OMe
- 25I-NB4OMe
Synthesis
25I-NBOMe is usually synthesised from 2C-I and 2-methoxybenzaldehyde, via reductive alkylation. It can be done stepwise by first making the imine and then reducing the formed imine with sodium borohydride, or by direct reaction with sodium triacetoxyborohydride.[2]
Society and culture
Legal status
Australia
25I-NBOMe was explicitly scheduled in Queensland drug law in April 2012, and in New South Wales in October 2013, as were some related compounds such as 25B-NBOMe. The Australian federal government has no specific legislation concerning any of the N-benzyl phenethylamines.[35]
Canada
As of October 31, 2016; 25I-NBOMe is a controlled substance (Schedule III) in Canada.[36]
China
As of October 2015 25I-NBOMe is a controlled substance in China.[37]
European Union
In September 2014 the European Union implemented a ban of 25I-NBOMe in all its member states.[38]
Israel
Israel banned 25I-NBOMe in 2013.[39]
Russia
Russia was the first country to pass specific regulations on the NBOMe series. All drugs in the NBOMe series, including 25I-NBOMe, became illegal in Russia in October 2011.[39]
United Kingdom
This substance is a Class A drug in the United Kingdom as a result of the N-benzylphenethylamine catch-all clause in the Misuse of Drugs Act 1971.[40]
United States
On Nov 15, 2013, the DEA added 25I-NBOMe (and 25C-, and 25B-NBOMe) to Schedule I using their emergency scheduling powers, making those NBOMe compounds "temporarily" in Schedule I for 2 years.[21] In November 2015, the temporary scheduling was extended for an additional year[41] while permanent scheduling was arranged.[42] 25I-NBOMe, 25B-NBOMe and 25C-NBOMe are currently Schedule 1 Substances according to 21 CFR 1308.11(d).[43]
Romania
In 2011, Romania banned all psychoactive substances.[44]
Serbia
25I-NBOMe was put on the list of prohibited substances in March 2015.[45]
Sweden
The Riksdag added 25I-NBOMe to Narcotic Drugs Punishments Act under Swedish schedule I ("substances, plant materials and fungi which normally do not have medical use") as of August 1, 2013, published by Medical Products Agency (MPA) in regulation LVFS 2013:15 listed as 25I-NBOMe, and 2-(4-jodo-2,5-dimetoxifenyl)-N-(2-metoxibensyl)etanamin.[46]
Taiwan
Following the European rule from 2014, 25I-NBOMe was put in class 4 of prohibited substances.[47]
Brazil
All drugs in the NBOMe family, including 25I-NBOMe, are illegal.
References
- ↑ UK Home Office (2014). "The Misuse of Drugs Act 1971 (Ketamine etc.) (Amendment) Order 2014". UK Government.
- 1 2 Heim R (25 March 2003). "Synthese und Pharmakologie potenter 5-HT2A-Rezeptoragonisten mit N-2-Methoxybenzyl-Partialstruktur. Entwicklung eines neuen Struktur-Wirkungskonzepts" (in German). diss.fu-berlin.de. Retrieved 2013-05-10.
- ↑ Braden MR (2007). "Towards a biophysical understanding of hallucinogen action". Dissertation. Purdue University: 1–176.
- 1 2 Ettrup A, Palner M, Gillings N, Santini MA, Hansen M, Kornum BR, et al. (November 2010). "Radiosynthesis and evaluation of 11C-CIMBI-5 as a 5-HT2A receptor agonist radioligand for PET". Journal of Nuclear Medicine. 51 (11): 1763–70. doi:10.2967/jnumed.109.074021. PMID 20956470.
- ↑ Hansen M (16 December 2010). "Design and synthesis of selective serotonin receptor agonists for positron emission tomography imaging of the brain". Ph.D. Thesis. Det Farmaceutiske Fakultet, København. CiteSeerX 10.1.1.690.4529.
- ↑ "Erowid 25I-NBOMe Vault".
- ↑ Vanderbilt University Medical Center (9 April 2015). "Poison center warns against designer drug 'N-bomb'". ScienceDaily.
- ↑ Mackin, Teresa (October 9, 2012). Dangerous synthetic drug making its way across the country. Archived October 31, 2012, at the Wayback Machine WISH-TV
- ↑ Morgans J (2017-02-08). "Everything We Know About NBOMe and Why it's Killing People". Vice. Retrieved 2019-07-23.
- 1 2 Hastings D (May 6, 2013). "New drug N-bomb hits the street, terrifying parents, troubling cops". New York Daily News. Retrieved 7 May 2013.
- 1 2 3 Lawn W, Barratt M, Williams M, Horne A, Winstock A (August 2014). "The NBOMe hallucinogenic drug series: Patterns of use, characteristics of users and self-reported effects in a large international sample". Journal of Psychopharmacology. 28 (8): 780–8. doi:10.1177/0269881114523866. PMID 24569095. S2CID 35219099.
- 1 2 3 4 Rose SR, Poklis JL, Poklis A (March 2013). "A case of 25I-NBOMe (25-I) intoxication: a new potent 5-HT2A agonist designer drug". Clinical Toxicology. 51 (3): 174–7. doi:10.3109/15563650.2013.772191. PMC 4002208. PMID 23473462.
- ↑ Poklis JL, Raso SA, Alford KN, Poklis A, Peace MR (October 2015). "Analysis of 25I-NBOMe, 25B-NBOMe, 25C-NBOMe and Other Dimethoxyphenyl-N-[(2-Methoxyphenyl) Methyl]Ethanamine Derivatives on Blotter Paper". Journal of Analytical Toxicology. 39 (8): 617–23. doi:10.1093/jat/bkv073. PMC 4570937. PMID 26378135.
- 1 2 3 4 5 6 7 8 Iversen L (4 June 2013). "Temporary class drug order on benzofury and NBOMe compounds". Advisory Council on the Misuse of Drugs. Gov.Uk.
- 1 2 3 4 Hill SL, Doris T, Gurung S, Katebe S, Lomas A, Dunn M, et al. (July 2013). "Severe clinical toxicity associated with analytically confirmed recreational use of 25I-NBOMe: case series". Clinical Toxicology. 51 (6): 487–92. doi:10.3109/15563650.2013.802795. PMID 23731373. S2CID 41259282.
- 1 2 3 "2C-I-NBOMe (25I) Effects". Erowid.
- 1 2 "25I-NBOMe". Erowid.
- ↑ "2C-I-NBOMe (25I) Dose". Erowid.
- ↑ Areas-Holmblad L (2016-12-11). "Using 'n-bomb' can kill you ~ Drug Addiction Now". Addiction Now | Substance Abuse, Drug Addiction and Recovery News Source. Retrieved 2020-09-04.
- 1 2 "Fatalities / Deaths". Erowid.
- 1 2 "Three More Synthetic Drugs Become Illegal for at Least Two Years". United States Drug Enforcement Administration (DEA). 15 November 2013.
- ↑ Malisow C (13 March 2013). "Breaking Bad: Digital Drug Sales, Analog Drug Deaths". Houston Press.
- ↑ Martin N (1 November 2012). "21-year-old dies after one drop of new synthetic drug at Voodoo Fest". NOLA.com.
- ↑ "Fatal overdose case continues". Hibbing Daily Tribune. 5 March 2015.
- ↑ Giles K (28 April 2015). "After friend's OD death, teens get a reprieve". Star Tribune.
- ↑ Lowe LM, Peterson BL, Couper FJ (October 2015). "A Case Review of the First Analytically Confirmed 25I-NBOMe-Related Death in Washington State". Journal of Analytical Toxicology. 39 (8): 668–71. doi:10.1093/jat/bkv092. PMID 26378143.
- ↑ Rice S (12 September 2012). "New hallucinogenic drug 25B-NBOMe and 25I-NBOMe led to South Australian man's bizarre death". News.com.au. Australia.
- ↑ Feehan C (21 January 2016). "Powerful N-Bomb drug - responsible for spate of deaths internationally - responsible for hospitalisation of six in Cork". Irish Independent.
- ↑ Moya PR, Berg KA, Gutiérrez-Hernandez MA, Sáez-Briones P, Reyes-Parada M, Cassels BK, Clarke WP (June 2007). "Functional selectivity of hallucinogenic phenethylamine and phenylisopropylamine derivatives at human 5-hydroxytryptamine (5-HT)2A and 5-HT2C receptors". The Journal of Pharmacology and Experimental Therapeutics. 321 (3): 1054–61. CiteSeerX 10.1.1.690.3752. doi:10.1124/jpet.106.117507. PMID 17337633. S2CID 11651502.
- 1 2 Ettrup A, Hansen M, Santini MA, Paine J, Gillings N, Palner M, et al. (April 2011). "Radiosynthesis and in vivo evaluation of a series of substituted 11C-phenethylamines as 5-HT (2A) agonist PET tracers". European Journal of Nuclear Medicine and Molecular Imaging. 38 (4): 681–93. doi:10.1007/s00259-010-1686-8. PMID 21174090. S2CID 12467684.
- 1 2 3 Nichols DE, Frescas SP, Chemel BR, Rehder KS, Zhong D, Lewin AH (June 2008). "High specific activity tritium-labeled N-(2-methoxybenzyl)-2,5-dimethoxy-4-iodophenethylamine (INBMeO): a high-affinity 5-HT2A receptor-selective agonist radioligand". Bioorganic & Medicinal Chemistry. 16 (11): 6116–23. doi:10.1016/j.bmc.2008.04.050. PMC 2719953. PMID 18468904.
- ↑ Silva ME, Heim R, Strasser A, Elz S, Dove S (January 2011). "Theoretical studies on the interaction of partial agonists with the 5-HT2A receptor". Journal of Computer-Aided Molecular Design. 25 (1): 51–66. Bibcode:2011JCAMD..25...51S. CiteSeerX 10.1.1.688.2670. doi:10.1007/s10822-010-9400-2. PMID 21088982. S2CID 3103050.
- ↑ Halberstadt AL, Geyer MA (February 2014). "Effects of the hallucinogen 2,5-dimethoxy-4-iodophenethylamine (2C-I) and superpotent N-benzyl derivatives on the head twitch response". Neuropharmacology. 77: 200–7. doi:10.1016/j.neuropharm.2013.08.025. PMC 3866097. PMID 24012658.
- ↑ Braden MR, Parrish JC, Naylor JC, Nichols DE (December 2006). "Molecular interaction of serotonin 5-HT2A receptor residues Phe339(6.51) and Phe340(6.52) with superpotent N-benzyl phenethylamine agonists". Molecular Pharmacology. 70 (6): 1956–64. doi:10.1124/mol.106.028720. PMID 17000863. S2CID 15840304.
- ↑ "Poisons Standard October 2015". Australian Government. October 2015.
- ↑ "Regulations Amending the Food and Drug Regulations (Part J — 2C-phenethylamines)". Canada Gazette. 4 May 2016.
- ↑ "关于印发《非药用类麻醉药品和精神药品列管办法》的通知" (in Chinese). China Food and Drug Administration. 27 September 2015. Archived from the original on 1 October 2015. Retrieved 1 October 2015.
- ↑ "Council Implementing Decision (2014/688/EU)". Official Journal of the European Union. 1 October 2014.
- 1 2 "2C-I-NBOMe Legal Status". Erowid.
- ↑ "The Misuse of Drugs Act 1971 (Ketamine etc.) (Amendment) Order 2014". www.legislation.gov.uk.
- ↑ Drug Enforcement Administration (November 2015). "Schedules of Controlled Substances: Extension of Temporary Placement of Three Synthetic Phenethylamines in Schedule I. Final order" (PDF). Federal Register. 80 (219): 70657–9. PMID 26567439.
- ↑ "Schedules of Controlled Substances: Placement of Three Synthetic Phenethylamines Into Schedule I". Archived from the original on May 13, 2017. Retrieved May 15, 2017.
- ↑ "eCFR PART 1308—SCHEDULES OF CONTROLLED SUBSTANCES Schedule I."
- ↑ "Legea 194/2011 privind combaterea operatiunilor cu produse susceptibile de a avea efecte psihoactive, altele decat cele prevazute de acte normative in vigoare, republicata 2014". Drept OnLine.
- ↑ "Министарство здравља Републикe Србије".
- ↑ "Föreskrifter om ändring i Läkemedelsverkets föreskrifter (LVFS 2011:10) om förteckningar över narkotika" [Regulations amending the Medical Products Agency's regulations (LVFS 2011: 10) on lists of drugs] (PDF). Läkemedelsverkets (The Medical Products Agency) (in Swedish). 11 July 2013. Archived from the original (PDF) on 28 September 2013.
- ↑ "衛生福利部食品藥物管理署".