Carbamazepine
 | |
 | |
| Names | |
|---|---|
| Trade names | Tegretol, Temporol, Neurotol, others |
IUPAC name
| |
| Clinical data | |
| Drug class | Anticonvulsant[1] |
| Main uses | Epilepsy, neuropathic pain[1] |
| Side effects | Nausea, drowsiness[1] |
| WHO AWaRe | UnlinkedWikibase error: ⧼unlinkedwikibase-error-statements-entity-not-set⧽ |
| Pregnancy category |
|
| Routes of use | By mouth |
| Defined daily dose | 1 gram (rectal) or 1 gram (by mouth)[2] |
| External links | |
| AHFS/Drugs.com | Monograph |
| MedlinePlus | a682237 |
| Legal | |
| License data | |
| Legal status | |
| Pharmacokinetics | |
| Bioavailability | ~100%[3] |
| Protein binding | 70-80%[3] |
| Metabolism | Liver—by CYP3A4, to active epoxide form (carbamazepine-10,11 epoxide)[3] |
| Elimination half-life | 36 hours (single dose), 16-24 hours (repeated dosing)[3] |
| Excretion | Urine (72%), feces (28%)[3] |
| Chemical and physical data | |
| Formula | C15H12N2O |
| Molar mass | 236.274 g·mol−1 |
| 3D model (JSmol) | |
SMILES
| |
InChI
| |
Carbamazepine (CBZ), sold under the trade name Tegretol among others, is an anticonvulsant medication used primarily in the treatment of epilepsy and neuropathic pain.[1] It is used in schizophrenia along with other medications and as a second-line agent in bipolar disorder.[4][1] Carbamazepine appears to work as well as phenytoin and valproate for focal and generalized seizures.[5] It is not effective for absence or myoclonic seizures.[1]
Common side effects include nausea and drowsiness.[1] Serious side effects may include skin rashes, decreased bone marrow function, suicidal thoughts, or confusion.[1] It should not be used in those with a history of bone marrow problems.[1] Use during pregnancy may cause harm to the baby; however, stopping the medication in pregnant women with seizures is not recommended.[1] Its use during breastfeeding is not recommended.[1] Care should be taken in those with either kidney or liver problems.[1]
Carbamazepine was discovered in 1953 by Swiss chemist Walter Schindler.[6][7] It was first marketed in 1962.[8] It is available as a generic medication, and none of the forms are expensive.[9] It is on the World Health Organization's List of Essential Medicines.[10] The wholesale cost in the developing world is about US$0.07 to US$0.24 per day as of 2015.[11] In 2017, it was the 176th most commonly prescribed medication in the United States, with more than three million prescriptions.[12][13]
Medical uses
Carbamazepine is typically used for the treatment of seizure disorders and neuropathic pain.[1] It is used off-label as a second-line treatment for bipolar disorder and in combination with an antipsychotic in some cases of schizophrenia when treatment with a conventional antipsychotic alone has failed.[1][14] However, evidence does not support this usage.[15] It is not effective for absence seizures or myoclonic seizures.[1] Although carbamazepine may have a similar effectiveness (people continue on medication) and efficacy (medicine reduces seizure recurrence and improves remission) when compared to phenytoin and valproate the choice of medications should be considered for each person individually as further research is needed to determine which medication is most helpful for people with newly-onset seizures.[5]
In the United States, the FDA-approved medical uses are epilepsy (including partial seizures, generalized tonic-clonic seizures and mixed seizures), trigeminal neuralgia, and manic and mixed episodes of bipolar I disorder.[16]
The drug is also claimed to be effective for ADHD.[17]
As of 2014, a controlled release formulation was available for which there is tentative evidence showing fewer side effects and unclear evidence with regard to whether there is a difference in efficacy.[18]
The structurally related drugs, oxcarbazepine and eslicarbazepine acetate, both show similar interactions, adverse events, and mechanisim of action profiles.[19]
Dosage
The defined daily dose of carbamazepine is 1 gram by mouth or rectally.[2]
The typical dose for seizures in adults; however, ranges from 400 mg per day to 1,200 mg per day.[1] Lower doses of 200 mg per day may be used for trigeminal neuralgia and increased up to 200 mg three times per day.[1][20]
Side effects
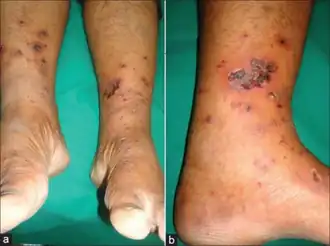
In the US, the label for carbamazepine contains warnings concerning:
- effects on the body's production of red blood cells, white blood cells, and platelets: rarely, there are major effects of aplastic anemia and agranulocytosis reported and more commonly, there are minor changes such as decreased white blood cell or platelet counts, but these do not progress to more serious problems.[3]
- increased risks of suicide[21]
- increased risks of hyponatremia and SIADH[3][22]
- risk of seizures, if the person stops taking the drug abruptly[3]
- risks to the fetus in women who are pregnant, specifically congenital malformations like spina bifida, and developmental disorders.[3][23]
Common adverse effects may include drowsiness, dizziness, headaches and migraines, motor coordination impairment, nausea, vomiting, and/or constipation. Alcohol use while taking carbamazepine may lead to enhanced depression of the central nervous system.[3] Less common side effects may include increased risk of seizures in people with mixed seizure disorders,[24] abnormal heart rhythms, blurry or double vision.[3] Also, rare case reports of an auditory side effect have been made, whereby patients perceive sounds about a semitone lower than previously; this unusual side effect is usually not noticed by most people, and disappears after the person stops taking carbamazepine.[25]
Interactions
Carbamazepine has a potential for drug interactions; caution should be used in combining other medicines with it, including other antiepileptics and mood stabilizers.[16] Lower levels of carbamazepine are seen when administrated with phenobarbital, phenytoin, or primidone, which can result in breakthrough seizure activity. Carbamazepine, as a CYP450 inducer, may increase clearance of many drugs, decreasing their concentration in the blood to subtherapeutic levels and reducing their desired effects.[26] Drugs that are more rapidly metabolized with carbamazepine include warfarin, lamotrigine, phenytoin, theophylline, and valproic acid.[16] Drugs that decrease the metabolism of carbamazepine or otherwise increase its levels include erythromycin,[27] cimetidine, propoxyphene, and calcium channel blockers.[16] Carbamazepine also increases the metabolism of the hormones in birth control pills and can reduce their effectiveness, potentially leading to unexpected pregnancies.[16] As a drug that induces cytochrome P450 enzymes, it accelerates elimination of many benzodiazepines and decreases their action.[28]
Valproic acid and valnoctamide both inhibit microsomal epoxide hydrolase (MEH), the enzyme responsible for the breakdown of carbamazepine-10,11 epoxide into inactive metabolites.[29] By inhibiting MEH, valproic acid and valnoctamide cause a build-up of the active metabolite, prolonging the effects of carbamazepine and delaying its excretion.
Grapefruit juice raises the bioavailability of carbamazepine by inhibiting CYP3A4 enzymes in the gut wall and in the liver.[3] Carbamazepine increases the processing of methadone resulting in lower blood levels.[30]
Pharmacogenetics
Serious skin reactions such as Stevens–Johnson syndrome or toxic epidermal necrolysis due to carbamazepine therapy are more common in people with a particular human leukocyte antigen allele, HLA-B*1502.[3] Odds ratios for the development of Stevens-Johnson syndrome or toxic epidermal necrolysis (SJS/TEN) in people who carry the allele can be in the double, triple or even quadruple digits, depending on the population studied.[31][32] HLA-B*1502 occurs almost exclusively in people with ancestry across broad areas of Asia, but has a very low or absent frequency in European, Japanese, Korean and African populations.[3][33] However, the HLA-A*31:01 allele has been shown to be a strong predictor of both mild and severe adverse reactions, such as the DRESS syndrome form of severe cutaneous reactions, to carbamazepine among Japanese, Chinese, Korean, and Europeans.[32][34] It is suggested that carbamazepine acts as a potent antigen that binds to the antigen-presenting area of HLA-B*1502 alike, triggering an everlasting activation signal on immature CD8-T cells, thus resulting in widespread cytotoxic reactions like SJS/TEN.[35]
Pharmacokinetics
Carbamazepine is relatively slowly but well absorbed after oral administration.[36] Its plasma half-life is about 36 hours when it is given as single dose, but it is a strong inducer of hepatic enzymes and the plasma half-life shortens to about 8-12 hours when it is given repeatedly.[36]
Mechanism of action
Carbamazepine is a sodium channel blocker.[37] It binds preferentially to voltage-gated sodium channels in their inactive conformation, which prevents repetitive and sustained firing of an action potential. Carbamazepine has effects on serotonin systems but the relevance to its antiseizure effects is uncertain. There is evidence that it is a serotonin releasing agent and possibly even a serotonin reuptake inhibitor.[38][39][40]
History
Carbamazepine was discovered by chemist Walter Schindler at J.R. Geigy AG (now part of Novartis) in Basel, Switzerland, in 1953.[41][42] It was first marketed as a drug to treat epilepsy in Switzerland in 1963 under the brand name "Tegretol"; its use for trigeminal neuralgia (formerly known as tic douloureux) was introduced at the same time.[41] It has been used as an anticonvulsant and antiepileptic in the UK since 1965, and has been approved in the US since 1968.[1]
In 1971, Drs. Takezaki and Hanaoka first used carbamazepine to control mania in patients refractory to antipsychotics (lithium was not available in Japan at that time). Dr. Okuma, working independently, did the same thing with success. As they were also epileptologists, they had some familiarity with the antiaggression effects of this drug. Carbamazepine was studied for bipolar disorder throughout the 1970s.[43]
Society and culture

Cost
The wholesale cost in the developing world is about US$0.07 to US$0.24 per day as of 2015.[11] In 2017, it was the 176th most commonly prescribed medication in the United States, with more than three million prescriptions.[12][13]
.svg.png.webp) Carbamazepine costs (US)
Carbamazepine costs (US).svg.png.webp) Carbamazepine prescriptions (US)
Carbamazepine prescriptions (US)
Environmental impact
Carbamazepine and its (bio-)transformation products have been detected in wastewater treatment plant effluent[44]: 224 and in streams receiving treated wastewater.[45] Field and laboratory studies have been conducted to understand the accumulation of carbamazepine in food plants grown in soil treated with sludge, which vary with respect to the concentrations of carbamazepine present in sludge and in the concentrations of sludge in the soil; taking into account only studies that used concentrations normally found, a 2014 review found that "the accumulation of carbamazepine into plants grown in soil amended with biosolids poses a de minimis risk to human health according to the approach."[44]: 227
Brand names
Carbamazepine is available worldwide under many brand names including Tegretol.[46]
References
- 1 2 3 4 5 6 7 8 9 10 11 12 13 14 15 16 17 18 "Carbamazepine". The American Society of Health-System Pharmacists. Archived from the original on 27 February 2015. Retrieved 28 March 2015.
- 1 2 "WHOCC - ATC/DDD Index". www.whocc.no. Archived from the original on 12 June 2020. Retrieved 26 August 2020.
- 1 2 3 4 5 6 7 8 9 10 11 12 13 14 "Carbamazepine Drug Label". Archived from the original on 8 December 2014.
- ↑ Nevitt SJ, Marson AG, Weston J, Tudur Smith C (August 2018). "Sodium valproate versus phenytoin monotherapy for epilepsy: an individual participant data review". The Cochrane Database of Systematic Reviews. 8: CD001769. doi:10.1002/14651858.CD001769.pub4. PMC 6513104. PMID 30091458.
- 1 2 Nevitt SJ, Marson AG, Tudur Smith C (July 2019). "Carbamazepine versus phenytoin monotherapy for epilepsy: an individual participant data review". The Cochrane Database of Systematic Reviews. 7: CD001911. doi:10.1002/14651858.CD001911.pub4. PMC 6637502. PMID 31318037.
- ↑ Smith, Howard S. (2009). Current therapy in pain. Philadelphia: Saunders/Elsevier. p. 460. ISBN 9781416048367. Archived from the original on 5 March 2016.
- ↑ US patent 2948718, Walter Schindler, "New n-heterocyclic compounds", published 1960-08-09, issued 1960-08-09, assigned to Geigy Chemical Corporation
- ↑ Moshé, Solomon (2009). The treatment of epilepsy (3 ed.). Chichester, UK: Wiley-Blackwell. p. xxix. ISBN 9781444316674. Archived from the original on 5 March 2016.
- ↑ Hitchings, Andrew; Lonsdale, Dagan; Burrage, Daniel; Baker, Emma (2019). The Top 100 Drugs: Clinical Pharmacology and Practical Prescribing (2nd ed.). Elsevier. pp. 102–103. ISBN 978-0-7020-7442-4. Archived from the original on 22 May 2021. Retrieved 9 November 2021.
- ↑ World Health Organization (2019). World Health Organization model list of essential medicines: 21st list 2019. Geneva: World Health Organization. hdl:10665/325771. WHO/MVP/EMP/IAU/2019.06. License: CC BY-NC-SA 3.0 IGO.
- 1 2 "Carbamazepine". International Medical Products Price Guide. Archived from the original on 29 July 2020. Retrieved 26 November 2019.
- 1 2 "The Top 300 of 2020". ClinCalc. Archived from the original on 12 February 2021. Retrieved 11 April 2020.
- 1 2 "Carbamazepine - Drug Usage Statistics". ClinCalc. Archived from the original on 28 February 2020. Retrieved 11 April 2020.
- ↑ Ceron-Litvoc D, Soares BG, Geddes J, Litvoc J, de Lima MS (January 2009). "Comparison of carbamazepine and lithium in treatment of bipolar disorder: a systematic review of randomized controlled trials". Human Psychopharmacology. 24 (1): 19–28. doi:10.1002/hup.990. PMID 19053079.
- ↑ Leucht S, Helfer B, Dold M, Kissling W, McGrath J (May 2014). Cochrane Schizophrenia Group (ed.). "Carbamazepine for schizophrenia". The Cochrane Database of Systematic Reviews (5): CD001258. doi:10.1002/14651858.CD001258.pub3. PMC 7032545. PMID 24789267.
- 1 2 3 4 5 Lexi-Comp (February 2009). "Carbamazepine". The Merck Manual Professional. Archived from the original on 3 November 2010. Retrieved on 3 May 2009.
- ↑ Millichap, J Gordon (1 March 1996). "Carbamazepine: A Therapy for ADHD". Pediatric Neurology Briefs. 10 (3): 20. doi:10.15844/pedneurbriefs-10-3-5.
- ↑ Powell G, Saunders M, Rigby A, Marson AG (December 2016). "Immediate-release versus controlled-release carbamazepine in the treatment of epilepsy". The Cochrane Database of Systematic Reviews. 12: CD007124. doi:10.1002/14651858.CD007124.pub5. PMC 6463840. PMID 27933615.
- ↑ Aledo-Serrano A, Gil-Nagel A (2020). Antiepileptic drugs: Carbamazepine, Oxcarbazepine and Eslicarbazepine Acetate. Springer Nature. Archived from the original on 28 August 2021. Retrieved 14 June 2020.
- ↑ "CARBAMAZEPINE oral - Essential drugs". medicalguidelines.msf.org. Archived from the original on 28 August 2021. Retrieved 26 August 2020.
- ↑ Jasdave S. Maan; Abdolreza Saadabadi. (2019). "Carbamazepine". Statpearls. 139 (1): 4–17. doi:10.1111/ane.13034. PMID 30291633. S2CID 52923061.
{{cite journal}}: CS1 maint: uses authors parameter (link) - ↑ Gandelman MS (March 1994). "Review of carbamazepine-induced hyponatremia". Progress in Neuro-Psychopharmacology & Biological Psychiatry. 18 (2): 211–33. doi:10.1016/0278-5846(94)90055-8. PMID 8208974. S2CID 36758508.
- ↑ Jentink J, Dolk H, Loane MA, Morris JK, Wellesley D, Garne E, de Jong-van den Berg L (December 2010). "Intrauterine exposure to carbamazepine and specific congenital malformations: systematic review and case-control study". BMJ. 341: c6581. doi:10.1136/bmj.c6581. PMC 2996546. PMID 21127116.
- ↑ Liu L, Zheng T, Morris MJ, Wallengren C, Clarke AL, Reid CA, et al. (November 2006). "The mechanism of carbamazepine aggravation of absence seizures". The Journal of Pharmacology and Experimental Therapeutics. 319 (2): 790–8. doi:10.1124/jpet.106.104968. PMID 16895979. S2CID 7693614.
- ↑ Tateno A, Sawada K, Takahashi I, Hujiwara Y (August 2006). "Carbamazepine-induced transient auditory pitch-perception deficit". Pediatric Neurology. 35 (2): 131–4. doi:10.1016/j.pediatrneurol.2006.01.011. PMID 16876011.
- ↑ "eMedicine - Toxicity, Carbamazepine". 2 February 2019. Archived from the original on 4 July 2008.
{{cite journal}}: Cite journal requires|journal=(help) - ↑ Stafstrom CE, Nohria V, Loganbill H, Nahouraii R, Boustany RM, DeLong GR (January 1995). "Erythromycin-induced carbamazepine toxicity: a continuing problem". Archives of Pediatrics & Adolescent Medicine. 149 (1): 99–101. doi:10.1001/archpedi.1995.02170130101025. PMID 7827672. Archived from the original on 18 November 2010.
- ↑ Moody D (2004). "Drug interactions with benzodiazepines". In Raymon LP, Mozayani A (eds.). Handbook of Drug Interactions: a Clinical and Forensic Guide. Humana. pp. 3–88. ISBN 978-1-58829-211-7.
- ↑ Gonzalez, Frank J.; Tukey, Robert H. (2006). "Drug Metabolism". In Brunton, Laurence; Lazo, John; Parker, Keith (eds.). Goodman & Gilman's The Pharmacological Basis of Therapeutics (11th ed.). New York: McGraw-Hill. p. 79. ISBN 978-0-07-142280-2.
- ↑ Schlatter J, Madras JL, Saulnier JL, Poujade F (September 1999). "[Drug interactions with methadone]" [Drug interactions with methadone]. Presse Médicale (in French). 28 (25): 1381–4. PMID 10506872.
{{cite journal}}: CS1 maint: unrecognized language (link) - ↑ Kaniwa N, Saito Y (June 2013). "Pharmacogenomics of severe cutaneous adverse reactions and drug-induced liver injury". Journal of Human Genetics. 58 (6): 317–26. doi:10.1038/jhg.2013.37. PMID 23635947.
- 1 2 Amstutz U, Shear NH, Rieder MJ, Hwang S, Fung V, Nakamura H, et al. (April 2014). "Recommendations for HLA-B*15:02 and HLA-A*31:01 genetic testing to reduce the risk of carbamazepine-induced hypersensitivity reactions". Epilepsia. 55 (4): 496–506. doi:10.1111/epi.12564. hdl:2429/63109. PMID 24597466.
- ↑ Leckband SG, Kelsoe JR, Dunnenberger HM, George AL, Tran E, Berger R, et al. (September 2013). "Clinical Pharmacogenetics Implementation Consortium guidelines for HLA-B genotype and carbamazepine dosing". Clinical Pharmacology and Therapeutics. 94 (3): 324–8. doi:10.1038/clpt.2013.103. PMC 3748365. PMID 23695185.
- ↑ Garon SL, Pavlos RK, White KD, Brown NJ, Stone CA, Phillips EJ (September 2017). "Pharmacogenomics of off-target adverse drug reactions". British Journal of Clinical Pharmacology. 83 (9): 1896–1911. doi:10.1111/bcp.13294. PMC 5555876. PMID 28345177.
- ↑ Jaruthamsophon, Kanoot; Tipmanee, Varomyalin; Sangiemchoey, Antida; Sukasem, Chonlaphat; Limprasert, Pornprot (30 March 2017). "HLA-B*15:21 and carbamazepine-induced Stevens-Johnson syndrome: pooled-data and in silico analysis". Scientific Reports. 7 (1): 45553. Bibcode:2017NatSR...745553J. doi:10.1038/srep45553. ISSN 2045-2322. PMC 5372085. PMID 28358139.
- 1 2 Rogawski, Michael A. (2020). "24. Antiseizure drugs". In Katzung, Bertram G.; Trevor, Anthony J. (eds.). Basic and Clinical Pharmacology (15th ed.). New York: McGraw-Hill. pp. 4427–430. ISBN 978-1-260-45231-0. Archived from the original on 10 October 2021. Retrieved 7 November 2021.
- ↑ Rogawski MA, Löscher W, Rho JM (May 2016). "Mechanisms of Action of Antiseizure Drugs and the Ketogenic Diet". Cold Spring Harbor Perspectives in Medicine. 6 (5): a022780. doi:10.1101/cshperspect.a022780. PMC 4852797. PMID 26801895.
- ↑ Dailey JW, Reith ME, Steidley KR, Milbrandt JC, Jobe PC (October 1998). "Carbamazepine-induced release of serotonin from rat hippocampus in vitro". Epilepsia. 39 (10): 1054–63. doi:10.1111/j.1528-1157.1998.tb01290.x. PMID 9776325.
- ↑ Dailey JW, Reith ME, Yan QS, Li MY, Jobe PC (June 1997). "Carbamazepine increases extracellular serotonin concentration: lack of antagonism by tetrodotoxin or zero Ca2+". European Journal of Pharmacology. 328 (2–3): 153–62. doi:10.1016/s0014-2999(97)83041-5. PMID 9218697.
- ↑ Kawata Y, Okada M, Murakami T, Kamata A, Zhu G, Kaneko S (June 2001). "Pharmacological discrimination between effects of carbamazepine on hippocampal basal, Ca(2+)- and K(+)-evoked serotonin release". British Journal of Pharmacology. 133 (4): 557–67. doi:10.1038/sj.bjp.0704104. PMC 1572811. PMID 11399673.
- 1 2 D.F. Scott. "Carbamazepine". Chapter 8 in The History of Epileptic Therapy: An Account of How Medication was Developed. History of Medicine Series. CRC Press, 1993 ISBN 9781850703914
- ↑ Schindler W, Häfliger F (1954). "Über Derivate des Iminodibenzyls". Helvetica Chimica Acta. 37 (2): 472–83. doi:10.1002/hlca.19540370211.
- ↑ Okuma T, Kishimoto A (February 1998). "A history of investigation on the mood stabilizing effect of carbamazepine in Japan". Psychiatry and Clinical Neurosciences. 52 (1): 3–12. doi:10.1111/j.1440-1819.1998.tb00966.x. PMID 9682927.
- 1 2 Prosser RS, Sibley PK (February 2015). "Human health risk assessment of pharmaceuticals and personal care products in plant tissue due to biosolids and manure amendments, and wastewater irrigation". Environment International. 75: 223–33. doi:10.1016/j.envint.2014.11.020. PMID 25486094.
- ↑ Posselt M, Jaeger A, Schaper JL, Radke M, Benskin JP (December 2018). "Determination of polar organic micropollutants in surface and pore water by high-resolution sampling-direct injection-ultra high performance liquid chromatography-tandem mass spectrometry". Environmental Science. Processes & Impacts. 20 (12): 1716–1727. doi:10.1039/C8EM00390D. PMID 30350841.
- ↑ "Carbamazepine". Drugs.com. Archived from the original on 5 October 2018. Retrieved 27 October 2019.
Further reading
- Iqbal MM, Gundlapalli SP, Ryan WG, Ryals T, Passman TE (March 2001). "Effects of antimanic mood-stabilizing drugs on fetuses, neonates, and nursing infants". Southern Medical Journal. 94 (3): 304–22. doi:10.1097/00007611-200194030-00007. PMID 11284518. Archived from the original on 27 July 2020. Retrieved 30 December 2019.
- Dean L (2015). "Carbamazepine Therapy and HLA Genotype". In Pratt VM, McLeod HL, Rubinstein WS, et al. (eds.). Medical Genetics Summaries. National Center for Biotechnology Information (NCBI). PMID 28520367. Bookshelf ID: NBK321445. Archived from the original on 26 October 2020. Retrieved 6 February 2020.
External links
| External sites: |
|
|---|---|
| Identifiers: |
|