MDPHP
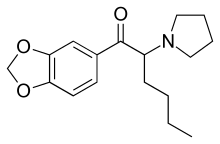
MDPHP (3',4'-Methylenedioxy-α-pyrrolidinohexiophenone) is a stimulant of the cathinone class originally developed in the 1960s,[1] which has been reported as a novel designer drug. In the UK its slang name is monkey dust.[2] It is closely related to the potent stimulant MDPV though with slightly milder effects, and has been used as an alternative in some countries following the banning of MDPV.[3][4][5][6]
 | |
 | |
| Legal status | |
|---|---|
| Legal status |
|
| Identifiers | |
IUPAC name
| |
| CAS Number | |
| PubChem CID | |
| ChemSpider | |
| UNII | |
| Chemical and physical data | |
| Formula | C17H23NO3 |
| Molar mass | 289.375 g·mol−1 |
| 3D model (JSmol) | |
SMILES
| |
InChI
| |
Legal Status
MDPHP is specifically listed as a controlled substance in Japan [7] and Hungary,[8] and is controlled under analogue provisions in a number of other jurisdictions.
See also
References
- DE 1545591, Koeppe H, Zeile K, Ludwig G, "Patent DE - Verfahren zur Herstellung von α-Aminoketonen mit heterocyclischer Aminogruppe", issued 28 May 1965
- "Monkey Dust drug use 'an epidemic', emergency workers warn". BBC. 10 August 2018. Retrieved 16 August 2018.
- Zaitsu K, Katagi M, Tsuchihashi H, Ishii A (2013). "Recently abused synthetic cathinones, α-pyrrolidinophenone derivatives: A review of their pharmacology, acute toxicity, and metabolism". Forensic Toxicology. 32: 1–8. doi:10.1007/s11419-013-0218-1. S2CID 25604845.
- Kaizaki-Mitsumoto A, Noguchi N, Yamaguchi S, Odanaka Y, Matsubayashi S, Kumamoto H, et al. (January 2016). "Three 25-NBOMe-type drugs, three other phenethylamine-type drugs (25I-NBMD, RH34, and escaline), eight cathinone derivatives, and a phencyclidine analog MMXE, newly identified in ingredients of drug products before they were sold on the drug market". Forensic Toxicology. 34 (1): 108–14. doi:10.1007/s11419-015-0293-6. S2CID 45890497.
- Beck O, Bäckberg M, Signell P, Helander A (April 2018). "Intoxications in the STRIDA project involving a panorama of psychostimulant pyrovalerone derivatives, MDPV copycats". Clinical Toxicology. Philadelphia, Pa. 56 (4): 256–263. doi:10.1080/15563650.2017.1370097. PMID 28895757. S2CID 3401681.
- Fowble KL, Shepard JR, Musah RA (March 2018). "Identification and classification of cathinone unknowns by statistical analysis processing of direct analysis in real time-high resolution mass spectrometry-derived "neutral loss" spectra". Talanta. 179: 546–553. doi:10.1016/j.talanta.2017.11.020. PMID 29310273.
- "指定薬物名称・構造式一覧(平成27年9月16日現在)" (PDF) (in Japanese). 厚生労働省. 16 September 2015. Retrieved 8 October 2015.
- "A Daath.hu kiegészítése a BSZKI "designer jogi listáján" nem szereplő, de a C-lista 1.-4. szerkezeti leírásainak megfelelő, illetve a C-lista 5. felsorolásában szereplő néhány anyagról" [The addition to Daath.hu is not included in the "designer legal list" of the BSZKI, but C-list 1.-4. and some of the substances in list 5 of list C.] (PDF) (in Hungarian).
This article is issued from Wikipedia. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.