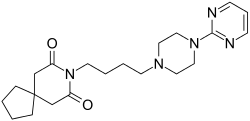
Buspirone
 | |
| Names | |
|---|---|
| Pronunciation | /ˈbjuːspɪroʊn/ (BEW-spi-rohn) |
| Trade names | Buspar, Namanspin |
| Other names | MJ 9022-1[1] |
IUPAC name
| |
| Clinical data | |
| Main uses | Anxiety disorders[2][3] |
| Side effects | Nausea, headaches, dizziness, trouble concentrating[2][4] |
| WHO AWaRe | UnlinkedWikibase error: ⧼unlinkedwikibase-error-statements-entity-not-set⧽ |
| Pregnancy category |
|
| Routes of use | By mouth |
| Defined daily dose | 30 mg[5] |
| External links | |
| AHFS/Drugs.com | Monograph |
| MedlinePlus | a688005 |
| Legal | |
| Legal status | |
| Pharmacokinetics | |
| Bioavailability | 3.9%[6] |
| Protein binding | 86–95%[7] |
| Metabolism | Liver (via CYP3A4)[8][9] |
| Metabolites | 5-OH-Buspirone; 6-OH-Buspirone; 8-OH-Buspirone; 1-PP[10][11][12] |
| Elimination half-life | 2.5 hours[8] |
| Excretion | Urine: 29–63%[7] Feces: 18–38%[7] |
| Chemical and physical data | |
| Formula | C21H31N5O2 |
| Molar mass | 385.512 g·mol−1 |
| 3D model (JSmol) | |
SMILES
| |
InChI
| |
Buspirone, sold under the brand name Buspar among others, is a medication primarily used to treat anxiety disorders, particularly generalized anxiety disorder.[2][3] Benefits support its short term use.[4] It has not been found to be effective in treating psychosis.[2] It is taken by mouth, and it may take up to four weeks for an effect.[2][3]
Common side effects include nausea, headaches, dizziness, and trouble concentrating.[2][4] Serious side effects may include hallucinations, serotonin syndrome, and seizures.[4] Use in pregnancy appears to be safe but has not been well studied, while use during breastfeeding is not recommended.[4][13] How it works is not clear but it is unrelated to benzodiazepines.[2]
Buspirone was first made in 1968 and approved for medical use in the United States in 1986.[2][3] It is available as a generic medication.[4] A month supply in the United Kingdom costs the NHS about £10 as of 2019.[4] In the United States the wholesale cost of this amount is about US$3.[14] In 2017, it was the 80th most commonly prescribed medication in the United States, with more than ten million prescriptions.[15][16]
Medical uses
Anxiety
Buspirone is used for the short-term treatment of anxiety disorders or symptoms of anxiety.[17][18][19][20][21] It is generally less preferred than selective serotonin reuptake inhibitors (SSRIs).[3]
Buspirone has no immediate anxiolytic effects, and hence has a delayed onset of action; its full clinical effectiveness may require 2–4 weeks to manifest.[22] The drug has been shown to be similarly effective in the treatment of generalized anxiety disorder (GAD) to benzodiazepines including diazepam, alprazolam, lorazepam, and clorazepate.[6] Buspirone is not known to be effective in the treatment of other anxiety disorders besides GAD,[23] although there is some limited evidence that it may be useful in the treatment of social phobia as an adjunct to selective serotonin reuptake inhibitors (SSRIs).[6][24]
Other uses
Sexual dysfunction
There is some evidence that buspirone on its own may be useful in the treatment of hypoactive sexual desire disorder (HSDD) in women.[25]
Miscellaneous
Buspirone is not effective as a treatment for benzodiazepine withdrawal, barbiturate withdrawal, or alcohol withdrawal/delirium tremens.[26]
SSRI and SNRI antidepressants such as paroxetine and venlafaxine may cause jaw pain/jaw spasm reversible syndrome (although it is not common), and buspirone appears to be successful in treating bruxism on SSRI/SNRI-induced jaw clenching.[27][28]
Dosage
The defined daily dose is 30 mg by mouth.[5]
Contraindications
Buspirone has these contraindications:[29][30]
- Hypersensitivity to buspirone
- Metabolic acidosis, as in diabetes
- Should not be used with MAO inhibitors
- Severely compromised liver or kidney function
Side effects
Known side effects associated with buspirone include dizziness, headaches, nausea, nervousness, and paresthesia.[6] Buspirone is relatively well tolerated, and is not associated with sedation, cognitive and psychomotor impairment, muscle relaxation, physical dependence, or anticonvulsant effects.[6] In addition, buspirone does not produce euphoria,[22] and is not a drug of abuse.[18]
It is unclear if there is a risk of tardive dyskinesia or other movement disorders with buspirone.[2]
Overdose
Buspirone appears to be relatively benign in cases of single-drug overdose, although no definitive data on this subject appear to be available.[31] In one clinical trial, buspirone was administered to healthy male volunteers at a dosage of 375 mg/day, and produced side effects including nausea, vomiting, dizziness, drowsiness, miosis, and gastric distress.[17][18][20] In early clinical trials, buspirone was given at dosages even as high as 2,400 mg/day, with akathisia, tremor, and muscle rigidity observed.[32] Deliberate overdoses with 250 mg and up to 300 mg buspirone have resulted in drowsiness in about 50% of individuals.[32] One death has been reported in association with 450 mg buspirone together with alprazolam, diltiazem, alcohol, cocaine.[32]
Interactions
Buspirone has been shown in vitro to be metabolized by the enzyme CYP3A4.[9] This finding is consistent with the in vivo interactions observed between buspirone and these inhibitors or inducers of cytochrome P450 3A4 (CYP3A4), among others:[29]
- Itraconazole: Increased plasma level of buspirone
- Rifampicin: Decreased plasma levels of buspirone
- Nefazodone: Increased plasma levels of buspirone
- Haloperidol: Increased plasma levels of haloperidol
- Carbamazepine: Decreased plasma levels of buspirone
- Grapefruit: Significantly increases the plasma levels of buspirone.[33] See grapefruit–drug interactions.
- Fluvoxamine: Moderately increase plasma levels of buspirone.[34]
Elevated blood pressure has been reported when buspirone has been administered to patients taking monoamine oxidase inhibitors (MAOIs).[29]
Pharmacology
Pharmacodynamics
| Site | Ki (nM) | Species | Ref |
|---|---|---|---|
| 5-HT1A | 3.98–214 21 (median) | Human | [35][36] |
| 5-HT1B | >100,000 | Rat | [37] |
| 5-HT1D | 22,000–42,700 | Human | [38][39] |
| 5-HT2A | 138 759–1,300 | Human Rat | [40] [37][40] |
| 5-HT2B | 214 | Human | [40] |
| 5-HT2C | 490 1,100–6,026 | Human Rat/pig | [40] [37][40] |
| 5-HT3 | >10,000 | Rat | [41][42] |
| 5-HT4 | >10,000 | Rat | [42] |
| 5-HT6 | 398 | Mouse | [43] |
| 5-HT7 | 375–381 | Rat | [44][45] |
| α1 | 1,000 | Rat | [37] |
| α2 | 6,000 | Rat | [46] |
| α2A | 7.3 (1-PP) | Human | [37] |
| β | 8,800 | Rat | [37] |
| D1 | 33,000 | Rat | [37] |
| D2 | 484 240 | Human Rat | [47] [37] |
| D3 | 98 | Human | [47] |
| D4 | 29 | Human | [47] |
| mACh | 38,000 | Rat | [37] |
| GABAA (BDZ) | >100,000 | Rat | [37] |
| Values are Ki (nM). The smaller the value, the more strongly the drug binds to the site. | |||
Buspirone acts as an agonist of the serotonin 5-HT1A receptor with high affinity.[6][37] It is a partial agonist of both presynaptic 5-HT1A receptors, which are inhibitory autoreceptors, and postsynaptic 5-HT1A receptors.[6] It is thought that the main effects of buspirone are mediated via its interaction with the presynaptic 5-HT1A receptor, thus reducing the firing of serotonin-producing neurons.[6] Buspirone also has lower affinities for the serotonin 5-HT2A, 5-HT2B, 5-HT2C, 5-HT6, and 5-HT7 receptors.[35]
In addition to binding to serotonin receptors, buspirone is an antagonist of the dopamine D2 receptor with weak affinity.[6][37] It preferentially blocks inhibitory presynaptic D2 autoreceptors, and antagonizes postsynaptic D2 receptors only at higher doses.[6] In accordance, buspirone has been found to increase dopaminergic neurotransmission in the nigrostriatal pathway at low doses, whereas at higher doses, postsynaptic D2 receptors are blocked and antidopaminergic effects such as hypoactivity and reduced stereotypy, though notably not catalepsy, are observed in animals.[6] Buspirone has also been found to bind with much higher affinity to the dopamine D3 and D4 receptors, where it is similarly an antagonist.[47]
A major metabolite of buspirone, 1-(2-pyrimidinyl)piperazine (1-PP), occurs at higher circulating levels than buspirone itself and is known to act as a potent α2-adrenergic receptor antagonist.[46][48][49] This metabolite may be responsible for the increased noradrenergic and dopaminergic activity observed with buspirone in animals.[48][50] In addition, 1-PP may play an important role in the antidepressant effects of buspirone.[50] Buspirone also has very weak and probably clinically unimportant affinity for the α1-adrenergic receptor.[37][51] However, buspirone has been reported to have shown "significant and selective intrinsic efficacy" at the α1-adrenergic receptor expressed in a "tissue- and species-dependent manner".[51]
Unlike benzodiazepines, buspirone does not interact with the GABAA receptor complex.[6][52]
Pharmacokinetics
Buspirone has a low oral bioavailability of 3.9% relative to intravenous injection due to extensive first-pass metabolism.[6] The time to peak plasma levels following ingestion is 0.9 to 1.5 hours.[6] It is reported to have an elimination half-life of 2.8 hours,[6] although a review of 14 studies found that the mean terminal half-life ranged between 2 and 11 hours, and one study even reported a terminal half-life of 33 hours.[10] Buspirone is metabolized primarily by CYP3A4, and prominent drug interactions with inhibitors and inducers of this enzyme have been observed.[8][9] Major metabolites of buspirone include 5-hydroxybuspirone, 6-hydroxybuspirone, 8-hydroxybuspirone, and 1-PP.[10][11][12] 6-Hydroxybuspirone has been identified as the predominant hepatic metabolite of buspirone, with plasma levels that are 40-fold greater than those of buspirone after oral administration of buspirone to humans.[11] The metabolite is a high-affinity partial agonist of the 5-HT1A receptor (Ki = 25 nM) similarly to buspirone, and has demonstrated occupancy of the 5-HT1A receptor in vivo.[11] As such, it is likely to play an important role in the therapeutic effects of buspirone.[11] 1-PP has also been found to circulate at higher levels than those of buspirone itself and may similarly play a significant role in the clinical effects of buspirone.[48][50]

Chemistry
Buspirone is a member of the azapirone chemical class, and consists of azaspirodecanedione and pyrimidinylpiperazine components linked together by a butyl chain.
Analogues
Structural analogues of buspirone include other azapirones like gepirone, ipsapirone, perospirone, and tandospirone.[55]
Synthesis
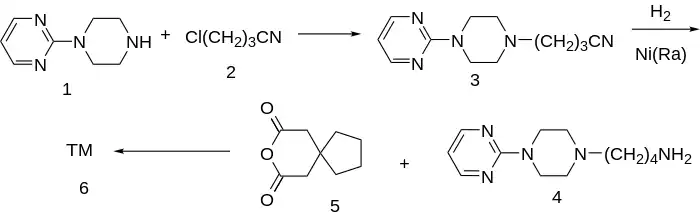
Alkylation of 1-(2-pyrimidyl)piperazine (1) with 3-chloro-1-cyanopropane (2, 4-chlorobutyronitrile) gives 3, which is reduced either by hydrogenation over Raney nickel catalyst, or with LAH. The resulting 1° amine (4) from the previous step is then reacted with 3,3-tetramethyleneglutaric anhydride (5, 8-Oxaspiro[4.5]decane-7,9-dione) in order to yield buspirone (6).
History
Buspirone was first synthesized, by a team at Mead Johnson, in 1968,[23] but was not patented until 1975.[56][57] It was initially developed as an antipsychotic drug acting on the D2 receptor, but was found to be ineffective in the treatment of psychosis and was repurposed as an anxiolytic.[6] In 1986, Bristol-Myers Squibb gained FDA approval for buspirone in the treatment of GAD.[23][58] The patent placed on buspirone expired in 2001 and it is now available as a generic drug.
Society and culture
Cost
A month supply in the United Kingdom costs the NHS about £10.00 as of 2019.[4] In the United States the wholesale cost of this amount is about US$2.65.[14] In 2017, it was the 80th most commonly prescribed medication in the United States, with more than ten million prescriptions.[15][16]
.svg.png.webp) Costs (US)
Costs (US).svg.png.webp) Prescriptions (US)
Prescriptions (US)
Generic names
Buspirone is the INN, BAN, DCF, and DCIT of buspirone, while buspirone hydrochloride is its USAN, BANM, and JAN.[1][59][60][61]
Brand name
Buspirone was primarily sold under the brand name Buspar.[59][61] Buspar is currently listed as discontinued by the US Federal Drug Administration.[62] In 2010, in response to a citizen petition, the US FDA determined that Buspar was not withdrawn for sale because of reasons of safety or effectiveness.[63]
2019 shortage
Due to interrupted production at a Mylan Pharmaceuticals plant in Morgantown, West Virginia, the United States experienced a shortage of buspirone in 2019.[64]
Research
Some tentative research supports other uses such as the treatment of depression and behavioral problems following brain damage.[6]
References
- 1 2 Elks J (14 November 2014). The Dictionary of Drugs: Chemical Data: Chemical Data, Structures and Bibliographies. Springer. pp. 192–. ISBN 978-1-4757-2085-3. Archived from the original on 12 March 2017. Retrieved 11 March 2017.
- 1 2 3 4 5 6 7 8 9 "Buspirone Hydrochloride Monograph for Professionals". Drugs.com. American Society of Health-System Pharmacists. Archived from the original on 6 March 2019. Retrieved 3 March 2019.
- 1 2 3 4 5 Wilson, TK; Tripp, J (January 2018). "Buspirone". StatPearls. PMID 30285372. Archived from the original on 2020-08-11. Retrieved 2019-03-05.
- 1 2 3 4 5 6 7 8 British national formulary : BNF 76 (76 ed.). Pharmaceutical Press. 2018. p. 338. ISBN 9780857113382.
- 1 2 "WHOCC - ATC/DDD Index". www.whocc.no. Archived from the original on 7 November 2020. Retrieved 6 September 2020.
- 1 2 3 4 5 6 7 8 9 10 11 12 13 14 15 16 17 Loane C, Politis M (June 2012). "Buspirone: what is it all about?". Brain Research. 1461: 111–8. doi:10.1016/j.brainres.2012.04.032. PMID 22608068.
- 1 2 3 "buspirone (Rx) - BuSpar, Buspirex, more." Medscape Reference. WebMD. Archived from the original on 13 July 2019. Retrieved 14 November 2013.
- 1 2 3 Mahmood I, Sahajwalla C (April 1999). "Clinical pharmacokinetics and pharmacodynamics of buspirone, an anxiolytic drug". Clinical Pharmacokinetics. 36 (4): 277–87. doi:10.2165/00003088-199936040-00003. PMID 10320950. Archived (PDF) from the original on 2021-08-28. Retrieved 2019-09-06.
- 1 2 3 4 Zhu M, Zhao W, Jimenez H, Zhang D, Yeola S, Dai R, et al. (April 2005). "Cytochrome P450 3A-mediated metabolism of buspirone in human liver microsomes". Drug Metabolism and Disposition. 33 (4): 500–7. doi:10.1124/dmd.104.000836. PMID 15640381. S2CID 10142905.
- 1 2 3 Gammans RE, Mayol RF, LaBudde JA (March 1986). "Metabolism and disposition of buspirone". The American Journal of Medicine. 80 (3B): 41–51. doi:10.1016/0002-9343(86)90331-1. PMID 3515929.
- 1 2 3 4 5 Schatzberg, Alan F.; Nemeroff, Charles B. (2009). The American Psychiatric Publishing Textbook of Psychopharmacology. American Psychiatric Pub. pp. 490–. ISBN 978-1-58562-309-9.
- 1 2 Wong H, Dockens RC, Pajor L, Yeola S, Grace JE, Stark AD, et al. (August 2007). "6-Hydroxybuspirone is a major active metabolite of buspirone: assessment of pharmacokinetics and 5-hydroxytryptamine1A receptor occupancy in rats". Drug Metabolism and Disposition. 35 (8): 1387–92. doi:10.1124/dmd.107.015768. PMID 17494642. S2CID 25558546.
- ↑ "Buspirone Pregnancy and Breastfeeding Warnings". Drugs.com. Archived from the original on 6 March 2019. Retrieved 3 March 2019.
- 1 2 "NADAC as of 2019-02-27". Centers for Medicare and Medicaid Services. Archived from the original on 2019-03-06. Retrieved 3 March 2019.
- 1 2 "The Top 300 of 2020". ClinCalc. Archived from the original on 12 February 2021. Retrieved 11 April 2020.
- 1 2 "Buspirone - Drug Usage Statistics". ClinCalc. Archived from the original on 8 July 2020. Retrieved 11 April 2020.
- 1 2 "BUSPIRONE HCL (buspirone hydrochloride) tablet [Watson Laboratories, Inc.]". DailyMed. Watson Laboratories, Inc. July 2013. Archived from the original on 13 June 2010. Retrieved 14 November 2013.
- 1 2 3 "BUSPAR® (buspirone hydrochloride) Tablets 5 mg & 10 mg PRODUCT INFORMATION" (PDF). TGA eBusiness Services. Aspen Pharma Pty Ltd. January 2010. Archived from the original on 24 September 2017. Retrieved 14 November 2013.
- ↑ Rossi S, ed. (2013). Australian Medicines Handbook (2013 ed.). Adelaide: The Australian Medicines Handbook Unit Trust. ISBN 978-0-9805790-9-3.
- 1 2 "Buspirone 10mg Tablets". electronic Medicines Compendium. Actavis UK Ltd. 10 September 2012. Archived from the original on 13 November 2013. Retrieved 14 November 2013.
- ↑ Joint Formulary Committee. British National Formulary (BNF). Pharmaceutical Press. p. 224.
- 1 2 Sadock, Benjamin J.; Sadock, Virginia A.; Ruiz, Pedro (22 September 2014). Kaplan and Sadock's Synopsis of Psychiatry: Behavioral Sciences/Clinical Psychiatry. Wolters Kluwer Health. pp. 3211–. ISBN 978-1-4698-8375-5. Archived from the original on 12 March 2017. Retrieved 11 March 2017.
- 1 2 3 Howland RH (November 2015). "Buspirone: Back to the Future". Journal of Psychosocial Nursing and Mental Health Services. 53 (11): 21–4. doi:10.3928/02793695-20151022-01. PMID 26535760.
- ↑ Masdrakis VG, Turic D, Baldwin DS (2013). "Pharmacological treatment of social anxiety disorder". Anxiety Disorders. Modern Trends in Pharmacopsychiatry. Vol. 29. pp. 144–53. doi:10.1159/000351960. ISBN 978-3-318-02463-0. PMID 25225024.
- ↑ Goldstein I, Kim NN, Clayton AH, DeRogatis LR, Giraldi A, Parish SJ, et al. (January 2017). "Hypoactive Sexual Desire Disorder: International Society for the Study of Women's Sexual Health (ISSWSH) Expert Consensus Panel Review". Mayo Clinic Proceedings. 92 (1): 114–128. doi:10.1016/j.mayocp.2016.09.018. PMID 27916394.
- ↑ Sontheimer DL, Ables AZ (March 2001). "Is imipramine or buspirone treatment effective in patients wishing to discontinue long-term benzodiazepine use?". The Journal of Family Practice. 50 (3): 203. PMID 11252203.
- ↑ Garrett AR, Hawley JS (April 2018). "SSRI-associated bruxism: A systematic review of published case reports". Neurology. Clinical Practice. 8 (2): 135–141. doi:10.1212/CPJ.0000000000000433. PMC 5914744. PMID 29708207.
- ↑ Prisco V, Iannaccone T, Di Grezia G (2017-04-01). "Use of buspirone in selective serotonin reuptake inhibitor-induced sleep bruxism". European Psychiatry. Abstract of the 25th European Congress of Psychiatry. 41: S855. doi:10.1016/j.eurpsy.2017.01.1701.
- 1 2 3 "Buspirone monograph". Drugs.com. Archived from the original on 2011-08-19. Retrieved 2011-08-27.
- ↑ Geddes, John; Gelder, Michael G.; Mayou, Richard (2005). Psychiatry. Oxford [Oxfordshire]: Oxford University Press. p. 237. ISBN 978-0-19-852863-0.
- ↑ Fulton B, Brogden RN (1997). "Buspirone". CNS Drugs. 7 (1): 68–88. doi:10.2165/00023210-199707010-00007. ISSN 1172-7047.
- 1 2 3 Dart, Richard C. (2004). Medical Toxicology. Lippincott Williams & Wilkins. pp. 886–. ISBN 978-0-7817-2845-4. Archived from the original on 2021-08-28. Retrieved 2017-09-24.
- ↑ Lilja JJ, Kivistö KT, Backman JT, Lamberg TS, Neuvonen PJ (December 1998). "Grapefruit juice substantially increases plasma concentrations of buspirone". Clinical Pharmacology and Therapeutics. 64 (6): 655–60. doi:10.1016/S0009-9236(98)90056-X. PMID 9871430.
- ↑ Lamberg TS, Kivistö KT, Laitila J, Mårtensson K, Neuvonen PJ (1998). "The effect of fluvoxamine on the pharmacokinetics and pharmacodynamics of buspirone". European Journal of Clinical Pharmacology. 54 (9–10): 761–6. doi:10.1007/s002280050548. PMID 9923581.
- 1 2 3 Roth BL, Driscol J. "PDSP Ki Database". Psychoactive Drug Screening Program (PDSP). University of North Carolina at Chapel Hill and the United States National Institute of Mental Health. Archived from the original on 28 August 2021. Retrieved 14 August 2017.
- ↑ Boess FG, Martin IL (1994). "Molecular biology of 5-HT receptors". Neuropharmacology. 33 (3–4): 275–317. doi:10.1016/0028-3908(94)90059-0. PMID 7984267.
- 1 2 3 4 5 6 7 8 9 10 11 12 13 Hamik A, Oksenberg D, Fischette C, Peroutka SJ (July 1990). "Analysis of tandospirone (SM-3997) interactions with neurotransmitter receptor binding sites". Biological Psychiatry. 28 (2): 99–109. doi:10.1016/0006-3223(90)90627-e. PMID 1974152.
- ↑ Peroutka SJ, Switzer JA, Hamik A (1989). "Identification of 5-hydroxytryptamine1D binding sites in human brain membranes". Synapse. 3 (1): 61–6. doi:10.1002/syn.890030109. PMID 2521959.
- ↑ Waeber C, Schoeffter P, Palacios JM, Hoyer D (June 1988). "Molecular pharmacology of 5-HT1D recognition sites: radioligand binding studies in human, pig and calf brain membranes". Naunyn-Schmiedeberg's Archives of Pharmacology. 337 (6): 595–601. doi:10.1007/bf00175783. PMID 2975354.
- 1 2 3 4 5 Bonhaus DW, Weinhardt KK, Taylor M, DeSouza A, McNeeley PM, Szczepanski K, et al. (1997). "RS-102221: a novel high affinity and selective, 5-HT2C receptor antagonist". Neuropharmacology. 36 (4–5): 621–9. doi:10.1016/s0028-3908(97)00049-x. PMID 9225287.
- ↑ Nelson DR, Thomas DR (May 1989). "[3H]-BRL 43694 (Granisetron), a specific ligand for 5-HT3 binding sites in rat brain cortical membranes". Biochemical Pharmacology. 38 (10): 1693–5. doi:10.1016/0006-2952(89)90319-5. PMID 2543418.
- 1 2 Borsini F, Giraldo E, Monferini E, Antonini G, Parenti M, Bietti G, Donetti A (September 1995). "BIMT 17, a 5-HT2A receptor antagonist and 5-HT1A receptor full agonist in rat cerebral cortex". Naunyn-Schmiedeberg's Archives of Pharmacology. 352 (3): 276–82. doi:10.1007/bf00168557. PMID 8584042.
- ↑ Plassat JL, Amlaiky N, Hen R (August 1993). "Molecular cloning of a mammalian serotonin receptor that activates adenylate cyclase". Molecular Pharmacology. 44 (2): 229–36. PMID 8394987.
- ↑ Lovenberg TW, Baron BM, de Lecea L, Miller JD, Prosser RA, Rea MA, et al. (September 1993). "A novel adenylyl cyclase-activating serotonin receptor (5-HT7) implicated in the regulation of mammalian circadian rhythms". Neuron. 11 (3): 449–58. doi:10.1016/0896-6273(93)90149-l. PMID 8398139.
- ↑ Ruat M, Traiffort E, Leurs R, Tardivel-Lacombe J, Diaz J, Arrang JM, Schwartz JC (September 1993). "Molecular cloning, characterization, and localization of a high-affinity serotonin receptor (5-HT7) activating cAMP formation". Proceedings of the National Academy of Sciences of the United States of America. 90 (18): 8547–51. Bibcode:1993PNAS...90.8547R. doi:10.1073/pnas.90.18.8547. PMC 47394. PMID 8397408.
- 1 2 Blier P, Curet O, Chaput Y, de Montigny C (July 1991). "Tandospirone and its metabolite, 1-(2-pyrimidinyl)-piperazine--II. Effects of acute administration of 1-PP and long-term administration of tandospirone on noradrenergic neurotransmission". Neuropharmacology. 30 (7): 691–701. doi:10.1016/0028-3908(91)90176-c. PMID 1681447.
- 1 2 3 4 Bergman J, Roof RA, Furman CA, Conroy JL, Mello NK, Sibley DR, Skolnick P (March 2013). "Modification of cocaine self-administration by buspirone (buspar®): potential involvement of D3 and D4 dopamine receptors". The International Journal of Neuropsychopharmacology. 16 (2): 445–58. doi:10.1017/S1461145712000661. PMC 5100812. PMID 22827916.
- 1 2 3 Tunnicliff G (September 1991). "Molecular basis of buspirone's anxiolytic action". Pharmacology & Toxicology. 69 (3): 149–56. doi:10.1111/j.1600-0773.1991.tb01289.x. PMID 1796057.
- ↑ Zuideveld KP, Rusiç-Pavletiç J, Maas HJ, Peletier LA, Van der Graaf PH, Danhof M (December 2002). "Pharmacokinetic-pharmacodynamic modeling of buspirone and its metabolite 1-(2-pyrimidinyl)-piperazine in rats". The Journal of Pharmacology and Experimental Therapeutics. 303 (3): 1130–7. doi:10.1124/jpet.102.036798. PMID 12438536. S2CID 14139919.
- 1 2 3 Fava M (2007). "The combination of buspirone and bupropion in the treatment of depression". Psychotherapy and Psychosomatics. 76 (5): 311–2. doi:10.1159/000104708. PMID 17700052.
- 1 2 Stern, Theodore A.; Fava, Maurizio; Wilens, Timothy E.; Rosenbaum, Jerrold F. (27 April 2015). Massachusetts General Hospital Psychopharmacology and Neurotherapeutics E-Book. Elsevier Health Sciences. pp. 29–. ISBN 978-0-323-41323-7.
- ↑ Nutt, David J.; Ballenger, James C. (15 April 2008). Anxiety Disorders. John Wiley & Sons. pp. 395–. ISBN 978-0-470-98683-7.
- ↑ Dockens RC, Salazar DE, Fulmor IE, Wehling M, Arnold ME, Croop R (November 2006). "Pharmacokinetics of a newly identified active metabolite of buspirone after administration of buspirone over its therapeutic dose range". Journal of Clinical Pharmacology. 46 (11): 1308–12. doi:10.1177/0091270006292250. PMID 17050795.
- ↑ Jajoo HK, Mayol RF, LaBudde JA, Blair IA (1989). "Metabolism of the antianxiety drug buspirone in human subjects". Drug Metabolism and Disposition. 17 (6): 634–40. PMID 2575499.
- ↑ Taylor DP, Moon SL (July 1991). "Buspirone and related compounds as alternative anxiolytics". Neuropeptides. 19 Suppl: 15–9. doi:10.1016/0143-4179(91)90078-w. PMID 1679210.
- 1 2 Allen LE, Ferguson HC, Kissel JW (May 1972). "Psychosedative agents. 2. 8-(4-Substituted 1-piperazinylalkyl)-8-azaspiro(4.5)decane-7,9-diones". Journal of Medicinal Chemistry. 15 (5): 477–9. doi:10.1021/jm00275a009. PMID 5035267.
- ↑ US Patent 3907801 N-(8 (4-pyridyl-piperazino)-alkyl(9 -azaspiroalkanediones
- ↑ United States Federal Drug Administration (September 9, 1986). Approval Type-1 New Molecular Entry. https://www.accessdata.fda.gov/drugsatfda_docs/nda/pre96/018731Orig1s000rev.pdf Archived 2019-09-20 at the Wayback Machine
- 1 2 Index Nominum 2000: International Drug Directory. Taylor & Francis. January 2000. pp. 149–. ISBN 978-3-88763-075-1. Archived from the original on 2017-03-12. Retrieved 2017-03-11.
- ↑ Morton IK, Hall JM (6 December 2012). Concise Dictionary of Pharmacological Agents: Properties and Synonyms. Springer Science & Business Media. pp. 57–. ISBN 978-94-011-4439-1. Archived from the original on 12 March 2017. Retrieved 11 March 2017.
- 1 2 "Buspirone". Archived from the original on 2017-03-12. Retrieved 2017-03-11.
- ↑ "Drugs@FDA: FDA Approved Drug Products". www.accessdata.fda.gov. Archived from the original on 2020-10-19. Retrieved 2019-09-20.
- ↑ "Determination That BUSPAR (Buspirone Hydrochloride) Tablets, 10 Milligrams, 15 Milligrams, and 30 Milligrams, Were Not Withdrawn From Sale for Reasons of Safety or Effectiveness". Federal Register. 2010-10-19. Archived from the original on 2019-09-20. Retrieved 2019-09-20.
- ↑ Rabin, Roni Caryn (2019-02-01). "Shortage of Anxiety Drug Leaves Patients Scrambling". The New York Times. ISSN 0362-4331. Archived from the original on 2019-09-03. Retrieved 2019-09-20.
External links
| External sites: |
|
|---|---|
| Identifiers: |