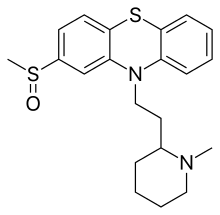
Mesoridazine
Mesoridazine (Serentil) is a piperidine neuroleptic drug belonging to the class of drugs called phenothiazines, used in the treatment of schizophrenia.[1] It is a metabolite of thioridazine. The drug's name is derived from the methylsulfoxy and piperidine functional groups in its chemical structure.
 | |
| Clinical data | |
|---|---|
| Trade names | Serentil |
| AHFS/Drugs.com | Micromedex Detailed Consumer Information |
| MedlinePlus | a682306 |
| Routes of administration | Oral, intravenous |
| Drug class | Typical antipsychotic |
| ATC code | |
| Legal status | |
| Legal status |
|
| Pharmacokinetic data | |
| Protein binding | 4% |
| Metabolism | Hepatic/renal |
| Elimination half-life | 24 to 48 hours |
| Excretion | Biliary and renal |
| Identifiers | |
IUPAC name
| |
| CAS Number | |
| PubChem CID | |
| IUPHAR/BPS | |
| DrugBank |
|
| ChemSpider |
|
| UNII | |
| KEGG |
|
| ChEBI | |
| ChEMBL | |
| CompTox Dashboard (EPA) | |
| Chemical and physical data | |
| Formula | C21H26N2OS2 |
| Molar mass | 386.57 g·mol−1 |
| 3D model (JSmol) | |
| Melting point | 130 °C (266 °F) |
| Solubility in water | insoluble mg/mL (20 °C) |
SMILES
| |
InChI
| |
| (verify) | |
It has central antiadrenergic, antidopaminergic, antiserotonergic and weak muscarinic anticholinergic effects.
Serious side effects include akathisia, tardive dyskinesia and the potentially fatal neuroleptic malignant syndrome.
Mesoridazine was withdrawn from the United States market in 2004 due to dangerous side effects, namely irregular heart beat and QT-prolongation of the electrocardiogram.[2]
It currently appears to be unavailable worldwide.
Synthesis
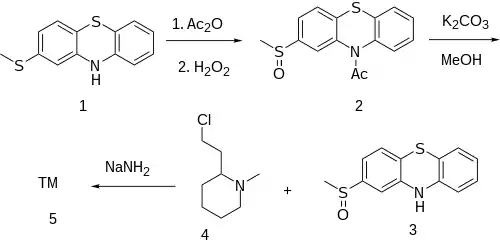
2-Methylthiophenothiazine [7643-08-5] (1) is treated with acetic anhydride] to give the protected amide, ie 10-acetyl-2-methylthiophenothiazine, CID:69367526. Oxidation of this by means of hydrogen peroxide and removal of the acetyl protecting group with potassium carbonate in methanol solution gives 2-methylsulfonylphenothiazine [23503-68-6] (3). Introduction of the sidechain by alkylation with 2-(2-chlorethyl)-1-methylpiperidine [50846-01-0] (6) in the presence of sodamide, afforded the desired mesoridazine (5).
References
- Gershon S, Sakalis G, Bowers PA (December 1981). "Mesoridazine -- a pharmacodynamic and pharmacokinetic profile". The Journal of Clinical Psychiatry. 42 (12): 463–9. PMID 7031039.
- American Society of Health-System Pharmacists (AHFS). "Mesoridazine". Medline Plus. U.S. National Library of Medicine.
- Bourquin, J.-P.; Schwarb, G.; Gamboni, G.; Fischer, R.; Ruesch, L.; Guldimann, S.; Theus, V.; Schenker, E.; Renz, J. (1958). "Synthesen auf dem Phenothiazin-Gebiet. 1. Mitteilung. Mercaptophenothiazin-Derivate". Helvetica Chimica Acta. 41 (4): 1061–1072. doi:10.1002/hlca.19580410419.
- Bourquin, J.-P.; Schwarb, G.; Gamboni, G.; Fischer, R.; Ruesch, L.; Guldimann, S.; Theus, V.; Schenker, E.; Renz, J. (1958). "Synthesen auf dem Phenothiazin-Gebiet. 2. Mitteilung. N-substituierte Mercaptophenothiazin-Derivate". Helvetica Chimica Acta. 41 (4): 1072–1108. doi:10.1002/hlca.19580410420.
- Schwarb Gustav, Renz Jany, Bourquin Jean-Pierre, U.S. Patent 3,084,161 (1963 to Sandoz Ltd).
| Typical |
|
|---|---|
| Disputed | |
| Atypical |
|
| Others |
|
| |
| Classes |
|
|---|---|
| Antidepressants (TCAs and TeCAs) |
|
| Antihistamines |
|
| Antipsychotics |
|
| Anticonvulsants | |
| Others |
|